
Filed pursuant to General Instruction II.L. of Form F-10
File No. 333-206994
A copy of this preliminary prospectus supplement has been filed with the securities regulatory authorities in the provinces of British Columbia, Alberta and Ontario, but has not yet become final for the purposes of the sale of securities. Information contained in this preliminary prospectus supplement may not be complete and may have to be amended.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise.
This prospectus supplement, together with the short form base shelf prospectus dated October 16, 2015 to which it relates, as amended or supplemented, and each document incorporated or deemed to be incorporated by reference in the short form base shelf prospectus, constitutes a public offering of securities offered pursuant hereto only in the jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities. See “Plan of Distribution.”
Information has been incorporated by reference in this prospectus supplement from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of Aurinia Pharmaceuticals Inc. at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia, Canada, V7X 1T2, telephone (604) 632-3473, and are also available electronically at www.sec.gov/edgar.shtml or www.sedar.com.
PRELIMINARY PROSPECTUS SUPPLEMENT No. 4
To the Short Form Base Shelf Prospectus Dated October 16, 2015
| New Issue | December 22, 2016 |

11,111,111 Units
This prospectus supplement and the accompanying short form base shelf prospectus dated October 16, 2015 qualifies the distribution (the “Offering”) of 11,111,111 units (each an “Offered Unit”) of Aurinia Pharmaceuticals Inc. (“Aurinia Pharmaceuticals”, the “Company”, “we”, “us” or “our”), at a price of US$2.25 per Offered Unit (the “Offering Price”). Each Offered Unit consists of one common share (each, a “Unit Share”) of the Company and one-half of one common share purchase warrant of the Company (each whole common share purchase warrant, a “Warrant”). The Offered Units will separate into Unit Shares and Warrants immediately upon distribution. Each Warrant will entitle the holder to purchase one common share of the Company (each a “Warrant Share”) at a price of US$3.00, subject to adjustment, at any time prior to 4:30 p.m. (New York time) on the date that is five years after the closing of the Offering. The Offered Units will be issued pursuant to an underwriting agreement (the “Underwriting Agreement”) dated December 22, 2016, between the Company, H.C. Wainwright & Co. LLC (the “Lead Underwriter”) and Cormark Securities Inc. (collectively, the “Underwriters”). The Offering Price was determined by negotiation between us and the Lead Underwriter on its own behalf and on behalf of the other Underwriter. The Offered Units will be offered in the United States and Canada through the Underwriters, either directly or through their duly registered U.S. or Canadian broker dealer affiliates or agents, as applicable. See “Plan of Distribution.”
Our common shares are listed and posted for trading on the Toronto Stock Exchange (the “TSX”) under the symbol “AUP” and on the NASDAQ Global Market (the “NASDAQ”) under the symbol “AUPH.” On December 21, 2016, the closing price of the common shares on the TSX was CDN$3.64 and the closing price of the common shares on the NASDAQ was US$2.72.
We have applied to list the Unit Shares and the Warrant Shares offered by this prospectus supplement on the TSX. Listing will be subject to our fulfillment of all of the requirements of the TSX. We have also applied to list the Unit Shares and the Warrant Shares offered by this prospectus supplement on the NASDAQ. Listing will be
subject to our fulfillment of all of the requirements of the NASDAQ. There will be no market in Canada or the United States through which the Warrants may be sold. This may affect the pricing of the Warrants in the Canadian or the United States secondary market, the transparency and availability of trading prices, the liquidity of such securities, and the extent of issuer regulation.
Investing in our securities involves a high degree of risk. You should carefully read the “Risk Factors” section in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein, as well as the information under the heading “Cautionary Note Regarding Forward Looking Information” in this prospectus supplement, and consider such notes and information in connection with an investment in any securities.
Price: US$2.25 per Unit
| Price to the Public(1) | Underwriters’ Fee(2) |
Net Proceeds to the Company(3) | ||||
| Per Unit |
US$2.25 | US$ | US$ | |||
| Total |
US$24,999,999.75 | US$ | US$ |
| (1) | The Company intends to allocate US$2.24 of the Offering Price as consideration for the issue of the Unit Share and US$0.01 of the Offering Price as consideration for the issue of the one-half of one Warrant comprising each Offered Unit. |
| (2) | In consideration for the services rendered by the Underwriters in connection with the Offering, the Underwriters will be paid an aggregate cash fee of US$ per Offered Unit, representing % of the gross proceeds of the Offering (the “Underwriters’ Fee”). See “Plan of Distribution.” |
| (3) | After deducting the Underwriters’ Fee, but before deducting expenses of the Offering, including in connection with the preparation and filing of this prospectus supplement, which are estimated to be US$450,000 and which will be paid from the proceeds of the Offering. Such net proceeds do not include any proceeds received from the exercise of the Warrants. |
The Company has granted the Underwriters an over-allotment option (the “Over-Allotment Option”), exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Underwriters, for a period of 30 days from the date of this prospectus supplement, to purchase up to an additional amount of Offered Units equal to 15% of the Offered Units sold pursuant to the Offering, being 1,666,666 Offered Units (the “Additional Offered Units”), at the Offering Price, less the Underwriters’ Fee, to cover over-allotments, if any, and for market stabilization purposes. The Over-Allotment Option may be exercisable by the Underwriters in respect of: (i) Additional Offered Units at the Offering Price; or (ii) additional Unit Shares (the “Additional Shares”) at a price of US$ per Additional Share; or (iii) additional Warrants (the “Additional Warrants”) at a price of US$ per Additional Warrant; or (iv) any combination of Additional Shares and/or Additional Warrants (together, the “Additional Securities”), so long as the aggregate number of Additional Shares and Additional Warrants which may be issued under the Over-Allotment Option does not exceed 1,666,666 Additional Shares and 833,333 Additional Warrants. The Additional Securities issuable upon exercise of the Over-Allotment Option are hereby qualified for distribution under this prospectus supplement. A purchaser who acquires Additional Securities issuable on the exercise of the Over-Allotment Option acquires such Additional Securities under this prospectus supplement regardless of whether the over-allotment position is ultimately filled through the exercise of the Over-Allotment Option or secondary market purchases. If the Over-Allotment Option is exercised in full, the total Price to the Public, Underwriters’ Fee and Net Proceeds to the Company (before payment of the expenses of the Offering and excluding any proceeds received from the exercise of the Warrants) will be approximately US$28.75 million, US$ million and US$ million, respectively. See “Plan of Distribution” and the table below:
| Underwriters’ Position |
Number of Additional Securities |
Exercise Period |
Exercise Price | |||
| Over-Allotment Option | 1,666,666 Additional Shares and/or 833,333 Additional Warrants |
30 days following the date of this prospectus supplement |
US$ per Additional Share and US$ per Additional Warrant |
Unless the context otherwise requires, all references to the “Offering” in this prospectus supplement shall include the Over-Allotment Option and all references to “Offered Units” shall include Additional Offered Units, references to “Unit Shares” shall include Additional Shares and references to “Warrants” shall include “Additional Warrants”, as applicable.
The Underwriters, as principal, conditionally offer the Offered Units subject to prior sale, if, as and when issued by the Company, and accepted by the Underwriters, in accordance with the conditions contained in the Underwriting Agreement described under “Plan of Distribution” and subject to the approval of certain legal matters on behalf of the Company by Borden Ladner Gervais LLP, with respect to Canadian legal matters, and by Cooley LLP, with respect to U.S. legal matters, and on behalf of the Underwriters by Stikeman Elliott LLP, with respect to Canadian legal matters, and by Goodwin Procter LLP, with respect to U.S. legal matters.
Subscriptions will be received subject to rejection or allotment in whole or in part and the right is reserved to close the subscription books at any time without notice. An electronic Deposit ID evidencing the Unit Shares is expected to be registered to CDS Clearing and Depository Services Inc. (“CDS”) and will be deposited with CDS at the closing of the Offering, which is anticipated to be on or about December 28, 2016 or such other date as may be agreed upon between the Company and the Underwriters. A purchaser of Unit Shares will receive only a customer confirmation from the registered dealer through which the Unit Shares comprising part of the Offered Units are purchased. Certificates representing the Warrants will be available for delivery to purchasers at closing of this Offering. The Company expects that delivery of the Offered Units will be made against payment therefor on or about the date of closing of the Offering, which is expected to be December 28, 2016. Investors who wish to trade Unit Shares or Warrants prior to the closing date of the Offering should consult their own advisors. See “Plan of Distribution.”
Entities affiliated with the ILJIN SNT Co., Ltd., one of our major stockholders, have submitted an indication of interest to purchase up to US$3.0 million of the Offered Units in this Offering. However, because indications of interest are not binding agreements or commitments to purchase, the Underwriters may determine to sell more, fewer or no Offered Units in this Offering to these entities, or these entities may determine to purchase more, fewer or no Offered Units in this Offering.
Investors should rely only on current information contained in or incorporated by reference into this prospectus supplement as such information is accurate only as of the date of the applicable document. The Company has not authorized anyone to provide investors with different information. Information contained on the Company’s website shall not be deemed to be a part of this prospectus supplement or incorporated by reference and should not be relied upon by prospective investors for the purpose of determining whether to invest in the securities. The Company will not make an offer of these securities in any jurisdiction where the offer or sale is not permitted. Investors should not assume that the information contained in this prospectus supplement is accurate as of any date other than the date on the face page of this prospectus supplement or the date of any documents incorporated by reference herein.
Gregory Ayers, Hyuek Joon Lee, David Jayne, Charles A. Rowland Jr. and Lorin Jeffry Randall, each a director of the Company, reside outside of Canada. Each of these directors has appointed Borden Ladner Gervais LLP, 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, as agent for service of process in Canada. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
In connection with the Offering, subject to applicable laws, the Underwriters may over-allot or effect transactions that stabilize or maintain the market price of the common shares at levels other than those which otherwise might prevail on the open market. Such transactions, if commenced, may be discontinued at any time. After the Underwriters have made a reasonable effort to sell all of the Offered Units offered by this prospectus supplement at the Offering Price specified herein, the Offering Price may be decreased, and further changed from time to time. Any such reduction will not affect the proceeds received by the Company. See “Plan of Distribution.”
H.C. Wainwright & Co., LLC is not registered as a dealer in the category of investment dealer in any Canadian jurisdiction and, accordingly, will only sell Offered Units into the United States and will not, directly or indirectly, solicit offers to purchase or sell the Offered Units in Canada.
We are permitted under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United States to prepare this prospectus supplement and the accompanying prospectus in accordance with the disclosure requirements of Canada. Prospective investors in the United States should be aware that such requirements are different from those of the United States. The financial statements incorporated by reference in this prospectus supplement and the accompanying prospectus have been prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, and are subject to Canadian auditing and auditor independence standards. As a result, our financial statements may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the acquisition of the securities described herein may have tax consequences both in Canada and the United States. Such consequences, for investors who are resident in, or citizens of, the United States, may not be described fully in this prospectus supplement, including the Canadian federal income tax consequences applicable to a foreign controlled Canadian corporation that acquires Offered Units. Investors should read the tax discussion in this prospectus supplement and the accompanying prospectus and consult their own tax advisors with respect to their own particular circumstances. See the sections titled “Certain Canadian Federal Income Tax Considerations,” “Material U.S. Federal Income Taxation Considerations” and “Risk Factors.”
Your ability to enforce civil liabilities under the United States federal securities laws may be affected adversely because we are incorporated in Canada, most of the officers and directors and some of the experts named in this prospectus supplement are not residents of the United States, and many of our assets and all or a substantial portion of the assets of such persons are located outside of the United States. See “Enforceability of Certain Civil Liabilities.”
Neither the U.S. Securities and Exchange Commission (the “SEC”) nor any state or Canadian securities regulator has approved or disapproved the securities offered hereby; passed upon the accuracy or adequacy of this prospectus supplement or determined if this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense.
Our registered office is located at #201, 17904 – 105 Avenue, Edmonton, Alberta T5S 2H5, Canada. Our head office is located at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8, Canada.
Sole Book-Running Manager
H.C. Wainwright & Co.
Co-Manager
Cormark Securities Inc.
The date of this prospectus supplement is , 2016
PROSPECTUS SUPPLEMENT
| S-1 | ||||
| S-1 | ||||
| S-3 | ||||
| S-6 | ||||
| S-8 | ||||
| S-8 | ||||
| S-9 | ||||
| S-20 | ||||
| S-21 | ||||
| S-25 | ||||
| S-26 | ||||
| S-27 | ||||
| S-28 | ||||
| S-30 | ||||
| S-31 | ||||
| DESCRIPTION OF SECURITIES OFFERED UNDER THIS PROSPECTUS SUPPLEMENT |
S-32 | |||
| S-34 | ||||
| S-38 | ||||
| S-43 | ||||
| S-50 | ||||
| S-50 | ||||
| S-51 | ||||
| S-52 | ||||
| S-52 | ||||
| S-52 | ||||
| S-53 | ||||
| C-1 | ||||
| C-2 |
PROSPECTUS
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 8 | ||||
| 20 | ||||
| 21 | ||||
| 21 | ||||
| 21 | ||||
| 22 | ||||
| 23 | ||||
| 24 | ||||
| 27 | ||||
| 27 | ||||
| 30 |
- i -
| 30 | ||||
| 30 | ||||
| 31 | ||||
| 32 | ||||
| 32 | ||||
| 32 | ||||
| 33 | ||||
| 34 |
- ii -
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of the securities we are offering and the method of distribution of those securities and also supplements and updates information regarding us contained in the accompanying base shelf prospectus. The second part, the accompanying prospectus, gives more general information about securities we may offer from time to time, some of which may not apply to the Offering. Both documents contain important information you should consider when making your investment decision. This prospectus supplement may add, update or change information contained in the accompanying prospectus. Before investing, you should carefully read both this prospectus supplement and the accompanying prospectus together with the additional information about us to which we refer you in the sections of this prospectus supplement titled “Documents Incorporated by Reference” and “Where You Can Find More Information.”
You should rely only on information contained in this prospectus supplement, the accompanying prospectus and the documents we incorporate by reference in this prospectus supplement and the accompanying prospectus. If information in this prospectus supplement is inconsistent with the accompanying prospectus or the information incorporated by reference, you should rely on this prospectus supplement. We have not authorized anyone to provide you with information that is different. If anyone provides you with any different or inconsistent information, you should not rely on it. We are offering the Offered Units only in jurisdictions where such offers are permitted by law. The information contained in this prospectus supplement and the accompanying prospectus is accurate only as of their respective dates, regardless of the time of delivery of this prospectus supplement and the accompanying prospectus and you should not assume otherwise.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference into this prospectus supplement and the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is part of a “shelf” registration statement on Form F-10 that we filed with the SEC. The shelf registration statement was declared effective by the SEC on November 5, 2015. This prospectus supplement does not contain all of the information contained in the registration statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC. You should refer to the registration statement and the exhibits to the registration statement for further information with respect to us and our securities.
In this prospectus supplement, unless stated otherwise or the context requires, all dollar amounts are expressed in U.S. dollars. All references to “$ or “US$” are to the lawful currency of the United States and all references to “CDN$” are to the lawful currency of Canada. This prospectus supplement and the documents incorporated by reference contain translations of some Canadian dollar amounts into U.S. dollars solely for your convenience. See the section titled “Exchange Rate Information.”
Market data and certain industry forecasts used in this prospectus supplement and the documents incorporated by reference herein or therein were obtained from market research, publicly available information and industry publications. We believe that these sources are generally reliable, but the accuracy and completeness of this information is not guaranteed. We have not independently verified such information, and we do not make any representation as to the accuracy of such information.
S-1
In this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein, unless the context otherwise requires, references to “we”, “us”, “our” or similar terms, as well as references to “Aurinia Pharmaceuticals” or the “Company”, refer to Aurinia Pharmaceuticals Inc., together with our subsidiaries.
This prospectus supplement is deemed to be incorporated by reference into the accompanying prospectus solely for the purposes of the Offering. Other documents are also incorporated or deemed to be incorporated by reference into this prospectus supplement and into the accompanying prospectus. See the section titled “Documents Incorporated by Reference.”
S-2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein contain “forward-looking statements” or “forward-looking information” within the meaning of applicable securities legislation. Forward-looking information is provided as of the date of this prospectus supplement and we do not intend, and do not assume any obligation, to update this forward-looking information, except as required by law.
A statement is forward-looking when it uses what we know and expect today to make a statement about the future. Forward-looking statements may include words such as “anticipate”, “believe”, “intend”, “expect”, “goal”, “may”, “outlook”, “plan”, “seek”, “should”, “strive”, “target”, “could”, “continue”, “potential” and “estimated”, or the negative of such terms or comparable terminology. You should not place undue reliance on the forward-looking statements, particularly those concerning anticipated events relating to the development, clinical trials, regulatory approval, and marketing of our products and the timing or magnitude of those events, as they are inherently risky and uncertain.
Securities laws encourage companies to disclose forward-looking information so that investors can get a better understanding of our future prospects and make informed investment decisions. These statements, made in this prospectus supplement or a document incorporated by reference herein, may include, among other things, statements with respect to:
| • | the Offering, including the terms, potential completion and expected closing date of the Offering and the intended use of proceeds of the Offering; |
| • | plans to fund our operations; |
| • | statements concerning strategic alternatives and future operations; |
| • | partnering activities; |
| • | summary statements relating to results of the past voclosporin trials or plans to advance the development of voclosporin; |
| • | statements concerning partnership activities and health regulatory discussions; |
| • | the timing of commencement and completion of clinical trials; |
| • | our intention to seek regulatory approvals in the United States and Europe for voclosporin; |
| • | our intention to seek additional corporate alliances and collaborative agreements to support the commercialization and development of our product; |
| • | our intention to demonstrate that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class status for the treatment of lupus nephritis (“LN”) outside of Japan; |
| • | our intention to initiate, and the timing of, the LN Phase 3 clinical trial; |
| • | our belief that recent granted formulation patents regarding the delivery of voclosporin to the ocular surface for conditions such as dry eye have the potential to be of therapeutic value; |
| • | our belief that voclosporin has further potential to be of therapeutic value in other autoimmune indications and in the prevention of transplant rejection; |
| • | our belief that the LN Phase 3 clinical trial will be de-risked based upon the AURA-LV results; |
| • | our belief in the market size and potential of lupus nephritis; |
| • | our intention to seek regulatory approval in other jurisdictions in the future and initiate clinical studies; |
| • | our anticipated future financial position, future revenues and projected costs; and |
| • | plans and objectives of management. |
S-3
Such statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based on a number of estimates and assumptions that, while considered reasonable by management, as at the date of such statements, are inherently subject to significant business, economic, competitive, political, scientific and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The factors and assumptions used by management to develop such forward-looking statements include, but are not limited to:
| • | the assumption that we will be able to reach agreements with regulatory agencies on executable development programs; |
| • | the assumption that recruitment to clinical trials will occur as projected; |
| • | the assumption that we will successfully complete our clinical programs on a timely basis, including conducting the required LN Phase 3 clinical trial and meet regulatory requirements for approval of marketing authorization applications and new drug approvals; |
| • | the assumption the regulatory requirements will be maintained; |
| • | the assumption that we will be able to manufacture and secure a sufficient supply of voclosporin to successfully complete the development and commercialization of voclosporin; |
| • | the assumption that our patent portfolio is sufficient and valid; |
| • | the assumption that there is a potential commercial value for other indications for voclosporin; |
| • | the assumption that market data and reports reviewed by us are accurate; |
| • | the assumption that our current good relationships with our suppliers, service providers and other third parties will be maintained; |
| • | the assumptions relating to the availability of capital on terms that are favourable to us; |
| • | the assumption that we will be able to attract and retain skilled staff; |
| • | the assumption that general business and economic conditions will be maintained; and |
| • | the assumptions relating to the feasibility of future clinical trials. |
It is important to know that:
| • | actual results could be materially different from what we expect if known or unknown risks affect our business, or if our estimates or assumptions turn out to be inaccurate. As a result, we cannot guarantee that any forward-looking statement will materialize and, accordingly, you are cautioned not to place undue reliance on these forward-looking statements; |
| • | forward-looking statements do not take into account the effect that transactions or non-recurring or other special items announced or occurring after the statements are made may have on our business. For example, they do not include the effect of mergers, acquisitions, other business combinations or transactions, dispositions, sales of assets, asset write-downs or other charges announced or occurring after the forward-looking statements are made. The financial impact of such transactions and non-recurring and other special items can be complex and necessarily depends on the facts particular to each of them. Accordingly, the expected impact cannot be meaningfully described in the abstract or presented in the same manner as known risks affecting our business; and |
| • | we disclaim any intention and assume no obligation to update any forward-looking statements even if new information becomes available, as a result of future events, new information, or for any other reason except as required by law. |
The factors discussed below and other considerations discussed in the “Risk Factors” section of this prospectus supplement, the accompanying prospectus, and the documents incorporated herein and therein, could cause our actual results to differ significantly from those contained in any forward-looking statements.
S-4
Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to differ materially from any further results, performance or achievements expressed or implied by such forward-looking statements. Important factors that could cause such differences include, among other things, the following:
| • | the need for additional capital in the longer term to fund our development programs and the effect of capital market conditions and other factors on capital availability; |
| • | difficulties, delays, or failures we may experience in the conduct of and reporting of results of our clinical trials for voclosporin; |
| • | difficulties in the manufacture and securing a sufficient supply of voclosporin on a timely basis to successfully complete the development and commercialization of voclosporin; |
| • | difficulties, delays or failures in obtaining regulatory approvals for the initiation of clinical trials; |
| • | difficulties, delays or failures in obtaining regulatory approvals to market voclosporin; |
| • | difficulties we may experience in completing the development and commercialization of voclosporin; |
| • | insufficient acceptance of and demand for voclosporin; |
| • | difficulties, delays, or failures in obtaining appropriate reimbursement from payors for voclosporin; and/or |
| • | difficulties we may experience in identifying and successfully securing appropriate corporate alliances to support the development and commercialization of our product. |
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These forward-looking statements are made as of the date of this prospectus supplement and we disclaim any intention and have no obligation or responsibility, except as required by law, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
S-5
DOCUMENTS INCORPORATED BY REFERENCE
This prospectus supplement is deemed to be incorporated by reference into the accompanying prospectus solely for the purposes of the Offering. Other documents are also incorporated, or are deemed to be incorporated by reference, into the accompanying prospectus and reference should be made to the accompanying prospectus for full particulars thereof.
Information has been incorporated by reference in this prospectus supplement from documents filed with the securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of Aurinia Pharmaceuticals Inc. at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, Canada, Telephone: (604) 632-3473, and are also available electronically at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC’s Electronic Data Gathering and Retrieval System (“EDGAR”) at www.sec.gov. Our filings through the System for Electronic Document Analysis and Retrieval (“SEDAR”) and through EDGAR are not incorporated by reference in this prospectus supplement, except as specifically set out herein.
The following documents, filed with the securities commissions or similar regulatory authorities in British Columbia, Alberta and Ontario, and filed with, or furnished to, the SEC are specifically incorporated by reference into, and form an integral part of, this prospectus supplement:
| (a) | our annual information form dated March 18, 2016 for the fiscal year ended December 31, 2015; |
| (b) | our audited consolidated balance sheets as at December 31, 2015 and 2014, and the consolidated statements of operations, changes in shareholders’ equity and cash flows for each of the years in the two-year period ended December 31, 2015, including the notes thereto and the auditors’ report thereon; |
| (c) | our unaudited comparative consolidated interim financial statements as at and for the three and nine month periods ended September 30, 2016 and 2015; |
| (d) | our Management’s Discussion and Analysis of Financial Condition and Results of Operations for the year ended December 31, 2015; |
| (e) | our Management’s Discussion and Analysis of Financial Condition and Results of Operations for the three month period ended March 31, 2016; |
| (f) | our Management’s Discussion and Analysis of Financial Condition and Results of Operations for the three and six month periods ended June 30, 2016; |
| (g) | our Management’s Discussion and Analysis of Financial Condition and Results of Operations for the three and nine month periods ended September 30, 2016; |
| (h) | our management information circular dated April 27, 2016 in connection with the annual general meeting of our shareholders held on June 8, 2016; |
| (i) | our material change report dated April 13, 2016 announcing a change in our chief executive officer position; |
| (j) | our material change report dated June 23, 2016 announcing the June 2016 Private Placement; |
| (k) | our material change report dated July 29, 2016 announcing the entering into of a controlled equity offering sales agreement with Cantor Fitzgerald & Co.; |
| (l) | our material change report dated August 22, 2016 announcing positive top-line results from the Phase 2b AURA-LV clinical trial; |
| (m) | our material change report dated November 9, 2016 announcing completion of the End of Phase 2 meeting with the U.S. Food & Drug Administration (“FDA”) and our plans to initiate a LN Phase 3 clinical trial; and |
S-6
| (n) | our material change report dated November 9, 2016 announcing the entry into of a controlled equity offering sales agreement with Cantor Fitzgerald & Co. |
Any document of the type referred to item 11.1 of Form 44-101F1 Short Form Prospectus under National Instrument 44-101 Short Form Prospectus Distributions of the Canadian Securities Administrators filed by Aurinia Pharmaceuticals with any securities commissions or similar regulatory authorities in Canada after the date of this prospectus supplement disclosing additional or updated information filed pursuant to the requirements of applicable securities legislation in Canada during the period that this prospectus supplement is effective shall be deemed to be incorporated by reference in this prospectus supplement. These documents are available on SEDAR, which can be accessed at www.sedar.com. In addition, to the extent that any document or information incorporated by reference in this prospectus supplement is included in a report that is filed or furnished to the SEC on Form 40-F, 20-F or 6-K (or any respective successor form), such document or information shall also be deemed to be incorporated by reference as an exhibit to the registration statement. In addition, if and to the extent indicated therein, we may incorporate by reference in this prospectus supplement, documents that we file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act.
Any statement contained in this prospectus supplement or in any document incorporated or deemed to be incorporated by reference in this prospectus supplement for the purpose of the Offering shall be deemed to be modified or superseded for the purposes of this prospectus supplement to the extent that a statement contained herein, or in any other subsequently filed document which also is incorporated or is deemed to be incorporated by reference herein, modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement will not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be deemed in its unmodified or superseded form to constitute a part of this prospectus supplement.
Upon filing a new annual information form and the related annual financial statements and management’s discussion and analysis with applicable securities regulatory authorities during the currency of this prospectus supplement, the previous annual information form, the previous annual financial statements and management’s discussion and analysis and all quarterly financial statements, supplemental information, material change reports and information circulars filed prior to the commencement of our financial year in which the new annual information form is filed will be deemed no longer to be incorporated into this prospectus supplement for purposes of future offers and sales of the securities under this prospectus supplement. Upon interim consolidated financial statements and the accompanying management’s discussion and analysis being filed by us with the applicable securities regulatory authorities during the duration of this prospectus supplement, all interim consolidated financial statements and the accompanying management’s discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer to be incorporated into this prospectus supplement for purposes of future offers and sales of securities under this prospectus supplement.
References to our website in any documents that are incorporated by reference into this prospectus supplement do not incorporate by reference the information on such website into this prospectus supplement or the accompanying prospectus, and we disclaim any such incorporation by reference.
S-7
Any “template version” of any “marketing materials” (as such terms are defined under applicable Canadian securities laws) that are used by the Underwriters in connection with the Offering are not part of this prospectus supplement to the extent that the contents of the template version of the marketing materials have been modified or superseded by a statement contained in this prospectus supplement. Any template version of any marketing materials that has been, or will be, filed under the Company’s profile on SEDAR at www.sedar.com before the termination of the distribution under the Offering (including any amendments to, or an amended version of, any template version of any marketing materials) is deemed to be incorporated by reference into this prospectus supplement.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
In addition to the documents specified in this prospectus supplement under the section titled “Documents Incorporated by Reference,” the following documents have been or will be (through post-effective amendment or incorporation by reference) filed with the SEC as part of the registration statement insofar as required by the SEC’s Form F-10: (i) the form of Underwriting Agreement with H.C. Wainwright & Co., LLC and Cormark Securities Inc. described in this prospectus supplement; (ii) powers of attorney from our directors and officers; and (iii) the consents of auditors and legal counsel.
S-8
The following description of the Company is derived from selected information about the Company contained in the documents incorporated by reference and does not contain all of the information about us and our business that should be considered before investing in the securities. This prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein should be reviewed and considered by prospective purchasers in connection with their investment in the Offered Units. This prospectus supplement may add to, update or change information in the accompanying prospectus. You should carefully read this entire prospectus supplement and the accompanying prospectus, including the risks and uncertainties discussed in the section titled “Risk Factors,” and the information incorporated by reference in this prospectus supplement, including our consolidated financial statements, before making an investment decision. If you invest in our securities, you are assuming a high degree of risk.
Name, Address and Incorporation
We are a clinical stage pharmaceutical company with a registered office located at #201, 17904 – 105 Avenue, Edmonton, Alberta T5S 2H5, Canada. Our head office is located at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8, Canada and incorporates our clinical, regulatory and business development functions.
We are organized under the Business Corporations Act (Alberta). Our common shares are currently listed and traded on the NASDAQ under the symbol “AUPH” and on the TSX under the symbol “AUP.”
Intercorporate Relationships
We have the following wholly-owned subsidiaries: Aurinia Pharma Corp. (British Columbia incorporated), Aurinia Pharmaceuticals, Inc. (Delaware incorporated) and Aurinia Pharma Limited (UK incorporated).
Business of the Company
We are focused on the development of our novel therapeutic immunomodulating drug candidate, voclosporin, for the treatment of LN. Voclosporin is a next generation calcineurin inhibitor (“CNI”) which has clinical data in over 2,000 patients across multiple indications. It has been studied in kidney rejection following transplantation, psoriasis and in various forms of uveitis (an ophthalmic disease).
Since September 20, 2013, we have rebranded, restructured and refocused around a strategy that focuses on the development of voclosporin for the treatment of LN. Voclosporin is an immunosuppressant, with a synergistic and dual mechanism of action that has the potential to improve near- and long-term outcomes in LN when added to mycophenolate mofetil (“MMF”), the current standard of care for LN. By inhibiting calcineurin, voclosporin blocks IL-2 expression and T-cell mediated immune responses. Voclosporin is made by a modification of a single amino acid of the cyclosporine molecule which has shown a more predictable pharmacokinetic and pharmacodynamic relationship, an increase in potency, an altered metabolic profile, and potential for flat dosing. Clinical doses of voclosporin studied to date range from 13 – 70 mg BID. The mechanism of action of voclosporin, a CNI, has been validated with certain first generation CNIs for the prevention of rejection in patients undergoing solid organ transplants and in several autoimmune indications, including dermatitis, keratoconjunctivitis sicca (Dry Eye Syndrome), psoriasis, rheumatoid arthritis, and for LN in Japan. We believe that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class regulatory approval status for the treatment of LN outside of Japan.
Based on published data, we believe the key benefits of voclosporin in the treatment of LN are as follows:
| • | Increased potency compared to cyclosporine A, allowing lower dosing requirements; |
| • | Limited inter and intra patient variability, allowing flat dosing; |
S-9
| • | Less cholesterolemia than cyclosporine A; and |
| • | Limited incidence of glucose intolerance and diabetes at targeted doses compared to tacrolimus. |
We are also pursuing out-license opportunities for our nanomicellar technology formulation patents for the delivery of voclosporin to the ocular surface for conditions such as dry eye to extract value from this intellectual property.
Lupus Nephritis (LN)
LN in an inflammation of the kidney caused by systemic lupus erythematosus (“SLE”) and represents a serious progression of SLE. SLE is a chronic, complex and often disabling disorder that affects over 1,000,000 people in the United States (mostly women). SLE is highly heterogeneous, affecting a wide range of organs and tissue systems. It is estimated that as many as 60% of all SLE patients have a form of LN that requires treatment. Unlike SLE, LN has straightforward disease measures where an early response correlates with long-term outcomes, measured by proteinuria. In patients with LN, renal damage results in proteinuria and/or hematuria and a decrease in renal function as evidenced by reduced estimated glomerular filtration rate (“eGFR”), and increased serum creatinine levels. Rapid control and reduction of proteinuria in lupus patients measured at 6 months shows a reduction in the need for dialysis at 10 years. LN can be debilitating and costly and if poorly controlled, can lead to permanent and irreversible tissue damage within the kidney. Recent literature suggests severe LN progresses to end-stage renal disease (“ESRD”), within 15 years of diagnosis in 10%-30% of patients, thus making LN a serious and potentially life-threatening condition. Mean annual medical cost for patients (both direct and indirect) with LN who progress to ESRD have been estimated to exceed US$60,000 per patient.
LN Standard of Care
While at Aspreva Pharmaceuticals, members of Aurinia’s management and clinical teams executed the Aspreva Lupus Management Study (“ALMS”) which established CellCept ®, or mycophenolate mofetil (“MMF”) as the current standard of care for treating LN. The ALMS study was published in 2009 in both the Journal of the American Society of Nephrology and The New England Journal of Medicine.
The American College of Rheumatology recommends that intravenous cyclophosphamide or MMF/CellCept® be used as first-line immunosuppressive therapy for LN. Despite their use, the ALMS study showed that the majority of patients failed to achieve Complete Remission (“CR”), or renal response at 24 weeks for both of these therapeutics. Based upon the results of the ALMS study, we believe that a better solution is needed to improve renal response rates for LN.
Based on the data outlined from the AURA Phase 2b clinical trial, we believe that voclosporin has the potential to address the critical need for LN by controlling active disease rapidly, lowering the steroid burden, impacting extra-renal disease and doing so with a convenient treatment regimen.
Market Potential and Commercial Considerations
We recently conducted our own market research which surveyed approximately 900 rheumatologists and nephrologists across the United States, Europe and Japan to outline the potential market size, pricing considerations and treatment paradigms in the United States, Europe and Japan. Using the U.S. MarketScan® data set (with approximately 170,000,000 insured lives in the United States) there were 445,346 SLE patients (between January 2006 and December 2015) based on specific SLE diagnosis codes. The National Institute of Diabetes and Digestive and Kidney Diseases estimates that up to 60% of people with SLE are diagnosed with LN. Using claims database research and additional physician research, we believe the diagnosed range of LN patients to be approximately 125,000 to 200,000 in the United States. The surveys also indicated that one in five LN patients are thought to be
S-10
undiagnosed due to referring physicians being inefficient and inaccurate in diagnosing the condition. Mean frequency of LN flares in otherwise controlled patients as reported by the surveyed physicians was approximately 14 months.
LN has a destructive cycle which is depicted below.
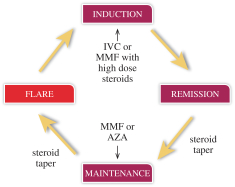
Based on the survey results, it is estimated that 58% of LN patients are controlled (maintenance phase); 25% poorly controlled and 17% have active disease (induction phase). Physicians indicated that if available, they would use voclosporin in a portion of patients in both the maintenance and induction phases. Only 18% of physicians were very satisfied or extremely satisfied with currently available and unapproved therapies’ ability to achieve a CR within 6 months.
Based on the pricing research we have conducted, we believe that the price range for voclosporin can be between US$50,000 and US$100,000 per patient per year in the United States. We believe that the U.S. market will provide the most opportunity and while the European population is likely larger than the United States, the pricing and market opportunity is more limited. We believe that the initial estimates of voclosporin peak sales may yield a global opportunity in excess of $1 billion (with greater than $1 billion in the United States; over $300 million in the European Union; and over $80 million Japan).
Recent Developments
Manufacturing Collaboration Agreement
We have entered into a long-term agreement with Lonza Ltd. (“Lonza”) for the manufacture of voclosporin active pharmaceutical ingredient (“API”). This agreement follows a successful multi-year clinical manufacturing relationship where the Company and Lonza have been refining the process and analytical methods to produce clinical and commercial supplies of voclosporin. Under the terms of the agreement, Lonza has agreed to produce cGMP-grade voclosporin drug substance for use in our Phase 3 LN clinical trial program and for future commercial use. The agreement also provides an option to have Lonza exclusively supply API for up to 20 years. We submitted a binding purchase order in the amount of CHF 2.05 million to Lonza for the manufacture of API for future use.
Appointment of New Director
On December 12, 2016, we announced the appointment of Lorin Jeffry “Jeff” Randall to our board of directors and Chairman of the Audit Committee. Mr. Randall currently serves on the boards of directors of Athersys, Inc., where he serves as Chairman of the Audit and Compensation Committees, and Acorda Therapeutics, Inc., where he serves on the Audit, Compliance and Nominations and Governance Committees.
Mr. Randall has over 30 years of experience serving in financial and operating roles spanning biotechnology, pharmaceuticals and manufacturing. He has led a number of companies through multi-million dollar financings
S-11
and mergers and acquisitions. In addition to his current board positions, Mr. Randall served on the board of directors of Nanosphere, Inc. from 2008 to 2016, most recently as Chairman of the Board. From 2004 to 2006, Mr. Randall, a financial consultant, was Senior Vice President and Chief Financial Officer of Eximias Pharmaceutical Corporation, a development-stage drug development company. Mr. Randall holds a B.S. in Mathematics and Accounting from Pennsylvania State University and an M.B.A. from Northeastern University.
FDA End of Phase 2 Meeting and Plans for Single LN Phase 3 Clinical Trial
On November 2, 2016, we announced the FDA’s preference for a single LN Phase 3 clinical trial for voclosporin in the treatment of LN, to be entitled “AURORA”. Pursuant to our recent End of Phase 2 meeting with the FDA Division of Pulmonary, Allergy and Rheumatology Products, we believe this LN Phase 3 clinical trial, the design of which is consistent with the ongoing AURA clinical trial, will, if successful, support a New Drug Application (“NDA”) submission.
The AURORA clinical trial will be a global 52-week double-blind, placebo controlled study of approximately 320 patients. We are finalizing the study protocol and regulatory submissions and in parallel are working on site selection with trial initiation anticipated in Q2 2017. Patients will be randomized 1:1 to either 23.7 mg of voclosporin BID and MMF or MMF and placebo, with both arms receiving a stringent oral corticosteroid taper. The study population will be comprised of patients with biopsy-proven active LN who will be evaluated on the primary efficacy endpoint of renal response at 24 weeks, a composite which includes:
| • | Urinary/protein creatinine ratio (“UPCR”) of £0.7 mg/mg |
| • | Normal, stable renal function (³60 mL/min/1.73 m2 or no confirmed decrease from baseline in eGFR of >20%) |
| • | Presence of sustained, low dose steroids (£10 mg prednisone from week 16-24) |
| • | No administration of rescue medications |
The readout of the primary endpoint of renal response at 24 weeks will occur after database lock at 52 weeks. Patients completing the 52 week study will then have the option to roll-over into a 104 week blinded continuation study. These data will allow us to assess long-term outcomes in LN patients that will be valuable in a post-marketing setting in addition to future interactions with various regulatory authorities.
While voclosporin has received fast track designation, the FDA has informed us that voclosporin is not eligible for breakthrough therapy designation at this time. We will continue to benefit from fast track designation, which includes more frequent communications with the FDA, potential for priority review of the NDA and an option to submit a rolling NDA submission, which may expedite the review process.
Our initial forecast is that the AURORA clinical trial will cost in the range of $70 million to $80 million. However, we are still in the process of obtaining quotes from suppliers and CROs and determining the optimum number of countries and sites in which to conduct the AURORA clinical trial and as a result this forecast may change. In addition, the initial estimate of the cost of the continuation study is in the range of $20 million to $25 million.
On December 13, 2016, we announced that we had received the final End of Phase 2 meeting minutes from the FDA Division of Pulmonary, Allergy and Rheumatology Products and that the minutes are consistent with the preliminary responses that were issued to us prior to the meeting which took place on October 25, 2016. We are currently having discussions with the European Medicines Agency (“EMA”) and the Japanese Pharmaceuticals and Medical Devices Agency (“PMDA”) regarding regulatory requirements for these jurisdictions.
S-12
AURION Clinical Trial Update
The AURION trial is a single-arm, twin center, exploratory study assessing the predictive value of an early reduction in proteinuria in subjects receiving 23.7mg of voclosporin BID with the current standard of care in patients with active LN. The primary objective of the AURION clinical trial is to examine biomarkers of disease activity at eight weeks and their ability to predict response at 24 and 48 weeks.
Study Design:
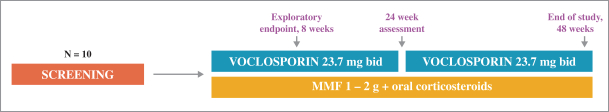
The primary analysis is the number of patients achieving each of the following biomarkers and the number of these patients who go on to achieve week 24 or week 48 remission.
Biomarkers:
| • | 25% reduction in urinary protein creatinine ratio (UPCR) at 8 weeks; |
| • | C3 complement normalization at 8 weeks; |
| • | C4 complement normalization at 8 weeks; and |
| • | Anti-dsDNA (double-stranded DNA) normalization at 8 weeks. |
The secondary analysis includes the 24 and 48 week outcomes, markers of SLE and pharmacokinetics and pharmacodynamics (PK/PD) of voclosporin.
On October 6, 2016, we announced 24 week data in all 10 patients from the AURION clinical trial, an open-label exploratory study to assess the short-term predictors of response using voclosporin (23.7 mg BID) in combination with MMF and oral corticosteroids in patients with active LN. The data was presented by Robert Huizinga, Vice President of Clinical Affairs at Aurinia Pharmaceuticals at the 10th Annual European Lupus Meeting in Venice, Italy.
The primary objective of the trial is to examine biomarkers of disease activity at eight weeks and their ability to predict response at 24 and 48 weeks.
In this trial, 70% (7/10) patients achieved complete remission (“CR”) at 24 weeks as measured by a UPCR of £ 0.5mg/mg, eGFR within 20% of baseline and concomitant steroid dose of <5 mg/day. Of the 10 patients that achieved a reduction of UPCR of ³ 25% at 8 weeks, 80% were responders (³ 50% reduction in UPCR over baseline) at 24 weeks and 70% were in CR at 24 weeks, proteinuria levels decreased by a mean of 61% from baseline through the first 24 weeks of the study. In addition, inflammatory markers such as C3, C4 and anti-dsDNA all continued to normalize to 24 weeks. Voclosporin was well-tolerated with no unexpected safety signals observed. Renal function, as measured by eGFR, also remained stable over the 24 weeks. We believe that the results of the AURION study supports the use of the 23.7 mg twice daily dose in further studies.
S-13
Details of the results are below:
| Patient # |
Attained
³25% UPCR at 8 weeks |
Attained Partial Remission* at 8 weeks |
Attained Partial Remission* at 24 weeks |
Attained CR at 8 weeks |
Attained CR at 24 weeks | |||||
| 1 |
Y | Y | Y | Y | Y | |||||
| 2 |
Y | Y | Y | Y | Y | |||||
| 3 |
Y | Y | Y | N | N | |||||
| 4 |
Y | N | N | N | N | |||||
| 5 |
Y | Y | Y | Y | Y | |||||
| 6 |
Y | Y | Y | Y | Y | |||||
| 7 |
Y | N | N | N | N | |||||
| 8 |
Y | Y | Y | Y | Y | |||||
| 9 |
Y | N | Y | N | Y | |||||
| 10 |
Y | Y | Y | N | Y | |||||
|
|
|
|
|
| ||||||
| TOTALS: |
100% (10/10) | 70% (7/10) | 80% (8/10) | 50% (5/10) | 70% (7/10) | |||||
|
|
|
|
|
|
| * | Retrospectively defined by ³50% reduction in UPCR |
AURA-LV (AURA) Phase 2b Clinical Trial – Positive Top-Line Results
On August 15, 2016, we announced positive top-line results from the Phase 2b AURA-LV (AURA) clinical trial in patients with active LN. The trial achieved its primary endpoint, demonstrating statistically significantly greater CR at 24 weeks (and confirmed at 26 weeks) in patients treated with 23.7 mg of voclosporin twice daily (p=0.045). This was the first global study of LN to meet its primary end point. Both treatment arms, 23.7 mg and 39.5 mg twice daily also showed a statistically significant improvement in the rate of achieving partial remission (“PR”) at 24 weeks (p=0.007; p=0.024). Each arm of the study included the current standard of care of MMF as background therapy, and a forced steroid taper.
AURA-LV Trial Design
The AURA–LV clinical trial or “Aurinia Urinary protein Reduction Active – Lupus with Voclosporin” compared the efficacy of voclosporin added to current standard of care of MMF, also known as CellCept®, against standard of care with placebo in achieving CR in patients with active LN. It enrolled 265 patients at centers in 20 countries worldwide. On entry to the trial, patients were required to have a diagnosis of LN according to established diagnostic criteria (American College of Rheumatology) and clinical and biopsy features indicative of active lupus nephritis.
Patients were randomized to one of two dosage groups of voclosporin (23.7 mg BID and 39.5 mg BID) or placebo, with all patients also receiving MMF and oral corticosteroids as background therapy. All patients had an initial IV dose of steroids (500-1000 mg) and then were started on 20-25 mg/daily, which was tapered down to a low dose of 5 mg daily by week 8 and 2.5 mg daily by week 16.
S-14
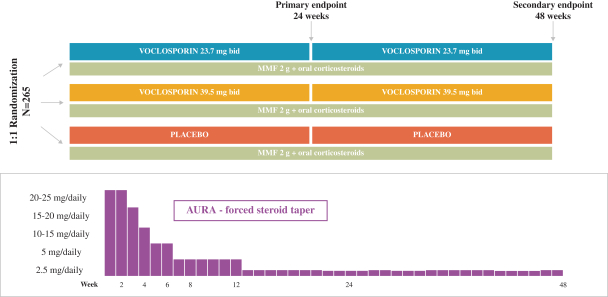
The primary endpoint was a measure of the number of patients who achieved CR at 24 weeks which had to be confirmed at 26 weeks. CR required the following four elements:
| • | protein/creatinine ratio of £0.5 mg/mg |
| • | normal stable renal function (eGFR ³60 mL/min/1.73m2 or no confirmed decrease from baseline in eGFR of ³20%) |
| • | Presence of sustained, low dose steroids (£10mg/day of prednisone from week 16 - 24) |
| • | No administration of rescue medications throughout the treatment period |
Summary of Results
The groups were generally well-balanced for age, gender and race, however, when considered together, the proteinuria and GFR data suggest that disease severity was greater for the low-dose voclosporin group.
Efficacy
| • | The primary endpoint of CR was met for the low-dose voclosporin group in the ITT analysis (p=0.045). 32.6% of patients on low dose achieved CR, compared to 27.3% on high dose and 19.3% in the control arm. |
| • | The odds ratio indicates that patients were twice as likely to achieve CR at 24 weeks compared to the control arm (OR=2.03). |
| • | The primary endpoint was re-analyzed using the 24-hour urine data in place of First Morning Void collections, confirming the finding that patients were twice as likely to achieve CR at 24 weeks compared to the control arm (p=0.047; OR=2.12). |
| • | Both voclosporin groups had a significantly faster time to CR (UPCR £ 0.5 mg/mg) than the control arm. Results of time to CR for co-variate analyses were broadly consistent with overall efficacy rates in those sub-groups. |
| • | The secondary endpoint of PR (50% reduction in UPCR over baseline with no administration of rescue medication throughout the treatment period) was met for both voclosporin groups in the ITT analysis with 69.7% of patients on low dose achieving PR (p=0.007) and 65.9% in the high dose group (p=0.024). 49.4% of patients in the control arm achieved PR. |
S-15
| • | Time to PR was similar (4 weeks) in the two voclosporin groups and was shorter than what was observed in the control group (6.6 weeks). |
Safety
| • | The overall rate of adverse events (“AEs”) was similar across all groups. |
| • | The overall rate of serious adverse events (“SAEs”) was higher in both voclosporin groups but the nature of SAEs is consistent with highly active LN. |
| • | The overall pattern of AEs and SAEs was consistent with that observed in other LN studies. |
| • | Overall renal function remained stable throughout the study period. |
| • | There were 13 deaths across the trial: two in the high-dose voclosporin arm; 10 in the low-dose voclosporin arm; and one in the control arm, with the majority of overall deaths (11/13) occurring in Asia. All deaths were assessed by the Investigator as being unrelated to study treatment. |
On September 29, 2016, we announced that in addition to voclosporin (23.7 mg BID) achieving its primary endpoint of CR at 24 weeks, both doses of voclosporin when added to the current standard of care of MMF and a forced oral corticosteroid taper have met all 24-week pre-specified secondary endpoints vs the control group. These pre-specified endpoints include: PR, which is measured by a ³50% reduction in UPCR with no concomitant use of rescue medication; time to CR and PR; reduction in Systemic Lupus Erythematosus Disease Activity Index or SLEDAI score; and reduction in UPCR over the 24-week treatment period.
| Pre-specified Secondary Endpoint |
Control | Low Dose VCS (23.7mg BID) |
High Dose VCS (39.5mg BID) | |||
| Time to Complete Remission (“TTCR”) [median] | Not achieved | 19.7 weeks | 23.4 weeks | |||
| p<.001 | p=.001 | |||||
| Partial Remission (as measured by UPCR reduction of ³ 50% from baseline) |
49% |
70% |
66% | |||
| p=.007 | p=.024 | |||||
| Time to Partial Remission (“TTPR”) [median] |
6.6 weeks |
4.1 weeks |
4.4 weeks | |||
| p=.002 | p=.003 | |||||
| SLEDAI Reduction |
-4.5 |
-6.3 |
-7.1 | |||
| p=.003 | p=.003 | |||||
| Reduction in UPCR |
-2.216 mg/mg |
-3.769 mg/mg |
-2.792 mg/mg | |||
| p<.001 | p=.006 | |||||
All p-values are vs control
On September 30, 2016, we presented detailed results on the AURA-LV Phase 2b clinical trial. These included a number of pre-specified subset and co-variate analyses and post-hoc analyses on the data, which show rapid proteinuria reduction and early remission. Based on recent literature suggesting that using a UPCR of £.7mg/mg has better predictive power regarding long-term renal outcomes in LN patients, we performed a post hoc analysis applying this measure. In doing so, we saw both a greater treatment difference between the 23.7mg BID voclosporin arm and the control arm, and better statistical power, which improves from a p-value of .045 to less than .01.
Based on these data we believe:
| • | voclosporin has shown statistically significant efficacy in multiple dimensions; |
| • | pre-specified and post-hoc analyses have provided valuable insight; |
S-16
| • | the LN Phase 3 clinical trial will be de-risked based upon the AURA-LV results; and |
| • | biomarker data suggest significant effect on the underlying immunologic process of the disease. |
We also released detailed safety data for the trial including an in-depth mortality assessment. The safety and tolerability of voclosporin has been well-documented in numerous studies. In previous studies, over 2,000 patients have been treated with voclosporin across multiple indications with no unexpected SAEs. Clinical doses of voclosporin studies to date range from 13-70 mg BID.
In comparing four global LN trials: AURA, Aspreva Lupus Management Study (ALMS), Ocrelizumab and Abatacept, it is evident that the AURA clinical trial enrolled the most severe patients, as measured by proteinuria at baseline. The difference in UPCR and the eGFR in the low dose voclosporin arm at baseline indicates patients had more severe disease.
No new safety signals were observed with the use of voclosporin in LN patients and voclosporin was well-tolerated. The overall safety profile of voclosporin is consistent with other immunomodulators. The summary of AEs by system organ class (SOC) across arms in the study is as follows:
| System Organ Class (SOC) |
Control N=88 |
Voclosporin 23.7mg BID N=89 |
Voclosporin 39.5mg BID N=88 | |||
| Any AE |
74 (84.1) |
81 (91.0) | 84 (95.5) | |||
Thirteen deaths have been reported in the AURA clinical trial—a pattern that is consistent with other global active LN studies. Eleven of thirteen deaths occurred at sites with compromised access to standard of care; and patients who died in the trial had a statistically different clinical baseline picture, indicating a more severe form of LN, potential comorbid conditions and poor nutrition. The last death in the study occurred in February 2016. Both the FDA and Data Safety Monitoring Board have reviewed in detail each death that occurred in the trial. The AURA clinical trial remains ongoing to its 48-week secondary endpoint.
On November 15, 2016, at the American College of Rheumatology annual meeting, we presented speed of remission data from the AURA trial in a late-breaking abstract titled “Speed of Remission with the Use of Voclosporin, MMF and Low Dose Steroids: Results of a Global Lupus Nephritis Study. The data presented are a post-hoc responder analysis (median time to CR for those who achieve CR), demonstrating 7.3 weeks to CR for voclosporin 23.7mg BID vs the control arm of 12 weeks.
On November 21, 2016, at the American Society of Nephrology Kidney Week 2016, we presented renal function data for the AURA trial in a late breaking session titled “High Impact Clinical Trials”. These data showed that in the voclosporin treatment arms, the renal function as measured by eGFR was stable and not significantly different from the control arm during the course of the trial. Mean blood pressure was slightly reduced and was similar between all treatment groups.
At-the-Market Facility – July 22, 2016
On July 22, 2016 we announced that we had entered into a controlled equity offering sales agreement with Cantor Fitzgerald & Co. pursuant to which the Company was authorized to sell, from time to time, through at-the-market offerings (the “July ATM”) with Cantor Fitzgerald & Co. acting as sales agent, such common shares as would have an aggregate offer price of up to US$10,000,000. We also filed a prospectus supplement with securities regulatory authorities in Canada in the provinces of British Columbia, Alberta and Ontario, and with the United States Securities and Exchange Commission, which supplemented our short form base shelf prospectus dated October 16, 2015, and our shelf registration statement on Form F-10 dated October 16, 2015, declared effective on November 5, 2015. Sales in the July ATM were only conducted in the United States through NASDAQ at market prices. No sales were conducted in Canada or through the Toronto Stock Exchange.
S-17
As of October 3, 2016, sales pursuant to the July ATM were concluded. We issued 3,306,085 common shares, receiving gross proceeds in the aggregate of US$8,000,000 ($6,142,000 in the third quarter of 2016 and $1,858,000 subsequent to the quarter end), (being the maximum value permissible in accordance with Canadian securities laws).
At-the-Market Facility – November 9, 2016
On November 9, 2016 we announced that we had entered into a controlled equity offering sales agreement with Cantor Fitzgerald & Co. pursuant to which the Company was authorized to sell, from time to time, through at-the-market offerings (the “November ATM”) with Cantor Fitzgerald & Co. acting as sales agent, such common shares as would have an aggregate offer price of up to US$8,000,000. We also filed a prospectus supplement with securities regulatory authorities in Canada in the provinces of British Columbia, Alberta and Ontario, and with the United States Securities and Exchange Commission, which supplemented our short form base shelf prospectus dated October 16, 2015, and our shelf registration statement on Form F-10 dated October 16, 2015, declared effective on November 5, 2015. Sales in the November ATM will only conducted in the United States through NASDAQ at market prices. No sales will be conducted in Canada or through the Toronto Stock Exchange.
As of December 21, 2016, we had issued 138,986 common shares and received gross proceeds of US$396,354.
Private Placement
On June 22, 2016, we completed a private placement (the “June 2016 Private Placement”) of 3,000,000 units (“Units”) at US$2.36 per Unit for aggregate gross proceeds of US$7,080,000. Each Unit consisted of one common share and a 0.35 of one common share purchase warrant exercisable for a period of two years from the date of issuance at an exercise price of US$2.77. Further information regarding the terms of the June 2016 Private Placement and the Units issued thereunder can be found in our material change report dated June 23, 2016, which is incorporated by reference herein.
Intellectual Property
Patents and other proprietary rights are essential to our business. Our policy has been to file patent applications to protect technology, inventions, and improvements to our inventions that we consider important to the development of our business.
As of September 30, 2016, we owned 11 granted United States patents and two United States patent applications related to cyclosporin analogs, including granted United States patents covering voclosporin composition of matter, methods of use, formulations and synthesis, which expire between 2018 and 2024, and 151 corresponding granted patents and four corresponding patent applications in other jurisdictions, excluding Canada, South Africa and Israel, which expire between 2018 and 2022. The corresponding Canadian, South African and Israeli patents are owned by Paladin Labs Inc. We anticipate that upon regulatory approval, patent protection for voclosporin will be extended in the United States and certain other major markets, including Europe and Japan, until at least October 2027 under the Hatch-Waxman Act and comparable laws in other countries. In addition to patent rights, we also expect to receive “new chemical entity” exclusivity for voclosporin in certain countries, which provides from five years in the United States to up to ten years in Europe of data exclusivity beyond the date of regulatory approval.
We have licensed the development and distribution rights to voclosporin for China, Hong Kong and Taiwan to 3Sbio Inc. This license is royalty bearing and we will also supply finished product to 3Sbio Inc. on a cost plus basis. We do not expect to receive any royalty revenue pursuant to this license in the foreseeable future.
S-18
As of September 30, 2016, we also owned two granted United States patents related to ophthalmic formulations of calcineurin inhibitors or mTOR inhibitors, including voclosporin, and one granted United States patent related to ophthalmic formulations of dexamethasone, which expire between 2028 and 2031. We also own 14 corresponding granted patents and four corresponding patent applications in other jurisdictions.
Near-term Events
We expect to continue our ongoing dialogue with the EMA and the PMDA to define the regulatory and clinical requirements to attain registration approvals in these jurisdictions over the next several months. Additionally, we expect to release the AURA 48 week results sometime during the first quarter of 2017. These results will be considered secondary end points and will include similar measures as compared to the already released 24 week end points. We also expect to enroll the first patients into the Phase 3 AURORA study sometime during the second quarter of 2017.
S-19
| Offered Units |
11,111,111 Units (or 12,777,777 Units if the Over-Allotment Option is exercised in full). Each Offered Unit consists of one Unit Share and one-half of one warrant. | |
| Terms of Warrants |
Each Warrant will entitle the holder to purchase one Warrant Share at a price of US$3.00 at any time prior to 4:30 p.m. (New York time) on the date that is five years after the closing of the Offering. | |
| Common Shares to be Outstanding After This Offering |
49,905,600 common shares (or 51,572,266 common shares if the Over-Allotment Option is exercised in full). | |
| Use of Proceeds |
The net proceeds from the Offering are expected to be approximately US$ million (or US$ million if the Over-Allotment Option is exercised in full) after deducting the Underwriters’ Fee of US$ million (or US$ million if the Over-Allotment Option is exercised in full) and our expenses of the Offering, which are estimated to be US$450,000 and excluding any proceeds received from the exercise of Warrants.
We expect to use the net proceeds of the for research and development activities including the LN Phase 3 clinical trial activities, and for working capital purposes. See the section titled “Use of Proceeds” on page S-30 of this prospectus supplement. | |
| Risk Factors |
Investing in our common shares involves a high degree of risk. Please read the information contained in and incorporated by reference under the section titled “Risk Factors” beginning on page S-21 of this prospectus supplement, and under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this prospectus supplement. | |
| NASDAQ Symbol |
“AUPH” | |
| TSX Symbol |
“AUP” | |
The number of common shares that will be outstanding immediately after this Offering as shown above is based on 38,794,489 common shares outstanding as of September 30, 2016. The number of shares outstanding as of September 30, 2016 as used throughout this prospectus supplement, unless otherwise indicated, excludes:
| • | 4,098,137 common shares issuable upon exercise of outstanding options outstanding as of September 30, 2016, with a weighted average exercise price of US$2.84; |
| • | 5,927,223 common shares issuable upon exercise of warrants outstanding as of September 30, 2016, with a weighted average exercise price of US$3.07; |
| • | 951,174 common shares reserved for future issuance under our stock option plans as of September 30, 2016; and |
| • | 1,235,971 common shares issued subsequent to September 30, 2016. |
Unless otherwise indicated, all information in this prospectus supplement assumes no exercise by the Underwriters of the Over-Allotment Option.
S-20
An investment in our securities is speculative and involves a high degree of risk. In addition to the other information included or incorporated by reference in this prospectus supplement and the accompanying prospectus, you should carefully consider the risks and uncertainties described under the heading “Risk Factors” in the accompanying prospectus, together with all of the other information contained in this prospectus supplement and the accompanying prospectus, before purchasing our securities. The occurrence of any of these risks could have a material adverse effect on our business, financial condition, results of operations and future prospects. In these circumstances, the market price of our common shares could decline, and you may lose all or part of your investment. These risks are not the only risks we face; risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business, financial condition and results of operations. Investors should also refer to the other information set forth or incorporated by reference in this prospectus supplement and the accompanying prospectus, including our consolidated financial statements and related notes. This prospectus supplement also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of a number of factors. See the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Risks Relating to the Offering
An investment in the Offered Units may result in the loss of an investor’s entire investment.
An investment in the Offered Units is speculative and may result in the loss of an investor’s entire investment. Only potential investors who are experienced in high risk investments and who can afford to lose their entire investment should consider an investment in Aurinia Pharmaceuticals.
No market for the Warrants currently exists.
The Company has applied to list the Unit Shares and the Warrant Shares on the TSX. Listing will be subject to the Company fulfilling all of the requirements of the TSX. The Company has also applied to list the Unit Shares and the Warrant Shares on the NASDAQ. Listing will be subject to the Company fulfilling all of the requirements of the NASDAQ. There will be no market in either Canada or United States through which the Warrants may be sold. This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices, the liquidity of such securities, and the extent of issuer regulation.
You will experience immediate and substantial dilution.
Since the price per share of the Offered Units is substantially higher than the net tangible book value per share of our common shares, you will suffer substantial dilution in the net tangible book value of the common shares you purchase in this Offering. Based on the public offering of 11,111,111 units at the price of US$2.25 per Offered Unit, after deducting Underwriters’ Fees and estimated Offering expenses payable by us, and based on a net tangible book value of our common shares as of September 30, 2016, as adjusted to give effect to this Offering, if you purchase units in this Offering, you will suffer immediate dilution of US$ per share in the net tangible book value of common shares. The exercise of outstanding stock options and warrants (including Warrants held by other purchasers) could result in further dilution of your investment. See the section titled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this Offering.
Since the Warrants are not listed on a national securities exchange or other nationally recognized trading system, the Underwriters are unable to satisfy any over-allotments without exercising the Underwriters’ Over-Allotment Option. Therefore, if the Underwriters exercise the Over-Allotment Option as to the Warrants in full, but not with respect to the Additional Shares, then the Warrant coverage will be higher than the initial 50% stated and you will be subject to further dilution. See the section titled “Dilution” in this prospectus supplement for a more detailed discussion of the dilution you will incur if you purchase Offered Units in this Offering.
S-21
Subsequent offerings will result in dilution to our shareholders.
We may sell additional equity securities in subsequent offerings (including through the sale of securities convertible into equity securities) and may issue additional equity securities to finance operations, acquisitions or other projects. We cannot predict the size of future issuances of equity securities or the size and terms of future issuances of debt instruments or other securities convertible into equity securities or the effect, if any, that future issuances and sales of our securities will have on the market price of our common shares. Any transaction involving the issuance of previously authorized but unissued common shares, or securities convertible into common shares, would result in dilution, possibly substantial, to securityholders. Exercises of presently outstanding share options may also result in dilution to securityholders.
Our board of directors has the authority to authorize certain offers and sales of additional securities without the vote of, or prior notice to, our shareholders. Based on the need for additional capital to fund expected expenditures and growth, it is likely that we will issue additional securities to provide such capital. Such additional issuances may involve the issuance of a significant number of our common shares at prices less than the current market price for the common shares.
Additionally, holders of stock options and warrants exercisable for our common shares (including other purchasers of Warrants) may elect to exercise their options or warrants into common shares. Such exercises could further dilute your investment.
The price of our common shares is subject to volatility, and your investment may suffer a decline in value.
The market prices for the securities of biotechnology companies, including ours, have historically been volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of any particular company.
The trading price of our common shares could continue to be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including the results and adequacy of our preclinical studies and clinical trials, as well as those of our collaborators, or our competitors; other evidence of the safety or effectiveness of our products or those of our competitors; announcements of technological innovations or new products by us or our competitors; governmental regulatory actions; developments with collaborators; developments (including litigation) concerning our patent or other proprietary rights of competitors; concern as to the safety of our products; period-to-period fluctuations in operating results; changes estimates of our performance by securities analysts; market conditions for biotechnology stocks in general; and other factors not within our control could have a significant adverse impact on the market price of our common shares, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A class action suit against us could result in substantial costs, potential liabilities and the diversion of management’s attention and resources.
There is no guarantee that an active trading market for our common shares will be maintained on the TSX and /or NASDAQ. Investors may not be able to sell their common shares quickly or at the latest market price if the trading in our common shares is not active.
We expect to issue common shares in the future. Future issuances of common shares, or the perception that such issuances are likely to occur, could affect the prevailing trading prices of the common shares. In addition, the existence of warrants may encourage short selling by market participants.
Sales of common shares could cause a decline in the market price of our common shares. Two of our major shareholders (venBio Global Strategic Fund, L.P. and ILJIN SNT Co., Ltd. and its affiliates) own an aggregate of approximately 29.54% of our outstanding common shares as at December 21, 2016. Any sales of common shares by these shareholders or other existing shareholders or holders of options may have an adverse effect on our ability to raise capital and may adversely affect the market price of our common shares. Entities affiliated with
S-22
the ILJIN SNT Co., Ltd. have submitted an indication of interest to purchase up to US$3.0 million of the Offered Units in this Offering. However, because indications of interest are not binding agreements or commitments to purchase, the Underwriters may determine to sell more, fewer or no Offered Units in this Offering to these entities, or these entities may determine to purchase more, fewer or no Offered Units in this Offering.
There is no assurance of a sufficient liquid trading market for our common shares in the future.
Our shareholders may be unable to sell significant quantities of common shares into the public trading markets without a significant reduction in the price of their common shares, or at all. There can be no assurance that there will be sufficient liquidity of our common shares on the trading market, and that we will continue to meet the listing requirements of the TSX or the NASDAQ or achieve listing on any other public listing exchange.
Future issuances of equity securities by us or sales by our existing shareholders may cause the price of the common shares to fall.
The market price of the common shares could decline as a result of issuances of securities or sales by our existing shareholders in the market, or the perception that these sales could occur. Sales of common shares by shareholders might also make it more difficult for us to sell common shares at a time and price that we deem appropriate. With an additional sale or issuance of common shares, investors will suffer dilution of their voting power and may experience dilution in earnings per share.
We will have broad discretion in the use of the net proceeds of the Offering and may not use them to effectively manage our business.
We will have broad discretion over the use of the net proceeds from this Offering. Because of the number and variability of factors that will determine our use of such proceeds, our ultimate use might vary substantially from our planned use. Investors may not agree with how we allocate or spend the proceeds from this Offering. We may pursue acquisitions, collaborations or clinical trials that do not result in an increase in the market value of the common shares, and may increase our losses.
We do not intend to pay dividends in the foreseeable future.
We have never declared or paid any dividends on our common shares. We intend, for the foreseeable future, to retain our future earnings, if any, to finance our commercial activities and further research and the expansion of our business. As a result, the return on an investment in common shares will likely depend upon any future appreciation in value, if any, and on a shareholder’s ability to sell common shares. The payment of future dividends, if any, will be reviewed periodically by our board of directors and will depend upon, among other things, conditions then existing including earnings, financial conditions, cash on hand, financial requirements to fund our commercial activities, development and growth, and other factors that our board of directors may consider appropriate in the circumstances.
We may be a passive foreign investment company, which may result in adverse U.S. federal income tax consequences for U.S. Holders.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes. Our status as a PFIC may also depend on how quickly we use the cash proceeds from this offering in our business. Based on the nature of our income and the value and composition of our assets, we do not believe we were a PFIC during 2015. While we also do not believe we will be a PFIC for the current taxable year, because PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, there can be no assurance that we will not be a PFIC for the current or future taxable years. If we are characterized as a
S-23
PFIC, our shareholders who are U.S. Holders (as defined in “Material U.S. Federal Income Taxation Considerations”) may suffer adverse tax consequences, including the treatment of gains realized on the sale of Unit Shares, Warrants and Warrant Shares as ordinary income, rather than as capital gain, the loss of the preferential rate applicable to dividends received on Unit Shares, Warrants and Warrant Shares by individuals who are U.S. Holders, and the addition of interest charges to the tax on such gains and certain distributions. A U.S. shareholder of a PFIC generally may mitigate these adverse U.S. federal income tax consequences by making a “qualified electing fund” election, or, to a lesser extent, a “mark to market” election. However, we do not intend to provide the information necessary for U.S. Holders to make qualified electing fund elections if we are classified as a PFIC. In addition, special PFIC rules may apply to Warrants and Warrant Shares acquired upon exercise of such Warrants, and certain of the elections described above may not be available with respect to Warrants and Warrant Shares. This paragraph is qualified in its entirety by the discussion in the section titled “Material U.S. Federal Income Taxation Considerations.”
You may be unable to enforce actions against us, certain of our directors and officers, or the experts named in this prospectus supplement under U.S. federal securities laws.
We are a corporation organized under the laws of Alberta, Canada. Most of our directors and officers, as well as the experts named in this prospectus supplement, reside principally in Canada or outside of the United States. Because all or a substantial portion of our assets and the assets of these persons are located outside of the United States, it may not be possible for investors to effect service of process within the United States upon us or those persons. Furthermore, it may not be possible for investors to enforce against us or those persons not in the United States, judgments obtained in U.S. courts based upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of the U.S. federal securities laws. Therefore, it may not be possible to enforce those actions against us, certain of our directors and officers or the experts named in this prospectus supplement.
S-24
CONSOLIDATED FINANCIAL INFORMATION AND CURRENCY
Our consolidated financial statements incorporated by reference in this prospectus supplement have been prepared in accordance with IFRS and are reported in United States dollars.
Unless stated otherwise or if the context otherwise requires, all references to dollar amounts in this prospectus supplement are references to U.S. dollars. References to “$” or “US$” are to U.S. dollars and references to “CDN$” are to Canadian dollars. In this prospectus supplement, where applicable, and unless otherwise indicated, amounts are converted from Canadian dollars to U.S. dollars and vice versa by applying the noon rate of exchange for conversion of one Canadian dollar to U.S. dollars as reported by the Bank of Canada on December 21, 2016.
The following table sets forth for each period indicated: (i) the noon exchange rates in effect at the end of the period; (ii) the high and low noon exchange rates during such period; and (iii) the average noon exchange rates for such period, for one Canadian dollar, expressed in U.S. dollars, as quoted by the Bank of Canada.
| Year Ended December 31, | ||||||||||||
| 2015 (US$) | 2014 (US$) | 2013 (US$) | ||||||||||
| Closing |
0.7225 | 0.8620 | 0.9402 | |||||||||
| High |
0.8527 | 0.9422 | 1.0164 | |||||||||
| Low |
0.7148 | 0.8589 | 0.9348 | |||||||||
| Average |
0.7820 | 0.9054 | 0.9710 | |||||||||
| Nine Months Ended September 30, | ||||||||||||
| 2016 (US$) | 2015 (US$) | 2014 (US$) | ||||||||||
| Closing |
0.7624 | 0.7493 | 0.8929 | |||||||||
| High |
0.7972 | 0.8527 | 0.9422 | |||||||||
| Low |
0.6854 | 0.7455 | 0.8888 | |||||||||
| Average |
0.7565 | 0.7936 | 0.9139 | |||||||||
On December 21, 2016, the noon exchange rate as quoted by the Bank of Canada was CDN$1.00 = US$0.7462.
S-25
Since September 30, 2016, the date of our most recently filed interim unaudited condensed consolidated financial statements, there have been no changes in our share capital on a consolidated basis other than as outlined under “Prior Sales.” For information on the issuance of common shares pursuant to a private placement completed on June 22, 2016, the July ATM, the November ATM, the exercise of options pursuant to our incentive stock option plan, and the exercise of certain outstanding warrants, see the section titled “Prior Sales.”
The following table sets forth our cash, cash equivalents and short term investments and capitalization as of September 30, 2016 on an actual basis and as adjusted to give effect to the common shares issued pursuant to the July ATM, the November ATM, the common shares issued upon the exercise of options and warrants to the date of this prospectus supplement and this Offering, as though they had occurred on such date. This table should be read in conjunction with our unaudited condensed consolidated interim financial statements as at and for the three and nine month periods ended September 30, 2016 and 2015. Figures are in thousands of U.S. dollars except share data.
| As at September 30, 2016 | ||||||||
| Actual | As Adjusted(1) |
|||||||
| Number of common shares issued and outstanding |
38,794,489 | 51,141,571 | (2) | |||||
|
|
|
|
|
|||||
| Cash, cash equivalents and short term investments |
US$ | 15,380 | US$ | |||||
|
|
|
|
|
|||||
| Derivative warrant liability |
US$ | 4,425 | US$ | 12,628 | (3) | |||
|
|
|
|
|
|||||
| Shareholders’ equity: |
||||||||
| Share capital – common shares |
275,805 | |||||||
| Warrants |
1,086 | 971 | ||||||
| Contributed surplus |
16,732 | 16,664 | ||||||
| Deficit |
(272,715 | ) | (272,715 | ) | ||||
| Accumulated other comprehensive loss |
(805 | ) | (805 | ) | ||||
|
|
|
|
|
|||||
| Total shareholders’ equity |
20,103 | |||||||
|
|
|
|
|
|||||
| Total capitalization |
US$ | 24,528 | US$ | |||||
|
|
|
|
|
|||||
| (1) | Excluding any exercise of the Over-Allotment Option and any proceeds we may receive from the exercise of the Warrants. |
| (2) | Change in common shares in the amount of 12,347,082 is comprised of shares issued: (a) pursuant to the July ATM – 687,903; (b) exercise of June 26, 2013 warrants – 122,222; (c) exercise of options – 30,000; (d) cashless exercise of 800,432 February 14, 2014 warrants – 256,860; (e) pursuant to the November ATM – 138,986; and (f) pursuant to this Offering – 11,111,111. |
| (3) | This amount includes the estimated fair value of $10.16 million calculated using the Black-Scholes pricing model allocated to Warrants issued pursuant to this Offering and is net of the fair value ($1.83 million) allocated to the 800,432 warrants exercised pursuant to the cashless exercise option at the time of exercise. |
S-26
Common Shares
Our common shares are listed and posted for trading on the TSX in Canada under the trading symbol “AUP” and on the NASDAQ in the United States under the trading symbol “AUPH.”
The following table sets forth, for the periods indicated, the reported high and low prices (in Canadian dollars) and volume of common shares traded for each month on the TSX.
| Month |
Price Range (CDN$) | Total Volume | ||||||||||
| High | Low | |||||||||||
| November 2015 |
3.94 | 3.13 | 98,699 | |||||||||
| December 2015 |
3.79 | 2.85 | 84,729 | |||||||||
| January 2016 |
3.76 | 2.45 | 158,052 | |||||||||
| February 2016 |
4.48 | 2.71 | 138,961 | |||||||||
| March 2016 |
4.25 | 2.80 | 238,709 | |||||||||
| April 2016 |
4.09 | 3.17 | 149,635 | |||||||||
| May 2016 |
3.75 | 2.82 | 125,467 | |||||||||
| June 2016 |
3.89 | 3.12 | 285,162 | |||||||||
| July, 2016 |
4.48 | 3.80 | 183,469 | |||||||||
| August 2016 |
5.83 | 2.23 | 1,985,935 | |||||||||
| September 2016 |
4.85 | 2.49 | 1,121,551 | |||||||||
| October 2016 |
7.50 | 4.06 | 2,274,556 | |||||||||
| November 2016 |
6.52 | 3.31 | 1,585,689 | |||||||||
| December 1 – 21, 2016 |
4.13 | 3.46 | 651,691 | |||||||||
At the close of business on December 21, 2016, the last trading day prior to the date of this prospectus supplement, the price of the common shares as quoted by the TSX was CDN$3.64.
The following table sets forth, for the periods indicated, the reported high and low prices (in United States dollars) and the volume of common shares traded for each month on NASDAQ.
| Month |
Price Range (US$) | Total Volume | ||||||||||
| High | Low | |||||||||||
| November 2015 |
3.05 | 2.34 | 672,961 | |||||||||
| December 2015 |
2.68 | 2.09 | 570,074 | |||||||||
| January 2016 |
2.69 | 1.42 | 677,264 | |||||||||
| February 2016 |
3.30 | 2.02 | 1,558,681 | |||||||||
| March 2016 |
3.25 | 2.05 | 1,068,958 | |||||||||
| April 2016 |
3.10 | 2.70 | 393,816 | |||||||||
| May 2016 |
3.00 | 2.16 | 580,808 | |||||||||
| June 2016 |
3.00 | 2.43 | 918,420 | |||||||||
| July, 2016 |
3.44 | 2.90 | 926,959 | |||||||||
| August 2016 |
4.49 | 1.74 | 44,368,734 | |||||||||
| September 2016 |
3.72 | 1.88 | 44,439,770 | |||||||||
| October 2016 |
5.69 | 2.99 | 78,162,253 | |||||||||
| November 2016 |
4.90 | 2.45 | 32,439,641 | |||||||||
| December 1 – 21, 2016 |
3.10 | 2.58 | 10,662,733 | |||||||||
At the close of business on December 21, 2016, the last trading day prior to the date of this prospectus supplement, the price of the common shares quoted by the NASDAQ was US$2.72.
S-27
Common Shares
The following table summarizes details of the common shares we issued during the 12 month period prior to the date of this prospectus supplement.
| Month of Issuance |
Security |
Price per Security |
Number of Securities |
|||||||
| June 2016 |
Units(1) | CDN$2.36 | 3,000,000 | |||||||
| August 2016 |
common shares(2) | CDN$3.50 | 10,000 | |||||||
| August 2016 |
common shares(3) | CDN$2.50 | 106,667 | |||||||
| August 2016 |
common shares(4) | US$2.51 | (5) | 1,148,545 | ||||||
| September 2016 |
common shares(4) | US$2.22 | (5) | 1,469,637 | ||||||
| September 2016 |
common shares(3) | CDN$2.25 | 32,000 | |||||||
| September 2016 |
common shares(3) | CDN$2.50 | 740,221 | |||||||
| October 2016 |
common shares(4) | US$2.70 | (5) | 687,903 | ||||||
| October 2016 |
common shares(2) | CDN$3.50 | 10,000 | |||||||
| October 2016 |
common shares(3) | CDN$2.50 | 11,111 | |||||||
| October 2016 |
common shares(6) | US$3.2204 | (6) | 223,703 | ||||||
| November 2016 |
common shares(2) | CDN$3.50 | 10,000 | |||||||
| November 2016 |
common shares(7) | US$3.2204 | (7) | 33,157 | ||||||
| November 2016 |
common shares(3) | CDN$2.50 | 111,111 | |||||||
| November 2016 |
common shares(8) | US$3.25 | (5) | 2,430 | ||||||
| December 2016 |
common shares(8) | US$2.84 | (5) | 136,556 | ||||||
| December 2016 |
common shares(2) | CDN$3.50 | 10,000 | |||||||
| Total |
7,743,041 | |||||||||
|
|
|
|||||||||
| (1) | Issued pursuant to the June 2016 Private Placement. Each Unit was comprised of one common share and 0.35 of a common share purchase warrant exercisable until June 26, 2018 at an exercise price of US$2.77. |
| (2) | Issued upon exercise of previously issued stock options. |
| (3) | Issued upon exercise of previously issued warrants. |
| (4) | Issued pursuant to at-the-market distribution pursuant to Prospectus Supplement No. 2 dated July 22, 2016. |
| (5) | Average selling price. |
| (6) | Issued pursuant to a cashless exercise option. Calculation was determined by the number of warrants to be exercised (618,516) multiplied by a five day weighted average market price (US$5.0451) less the exercise price (US$3.2204), with the difference divided by the weighted average market price. |
| (7) | Issued pursuant to a cashless exercise option. Calculation was determined by the number of warrants to be exercised (181,916) multiplied by a five day weighted average market price (US$3.9382) less the exercise price (US$3.2204), with the difference divided by the weighted average market price. |
| (8) | Issued pursuant to at-the-market distribution pursuant to Prospectus Supplement No. 3 dated November 9, 2016. |
S-28
Stock Options
The following table summarizes details of the stock options we granted during the 12 month period prior to the date of this prospectus supplement.
|
|
Security |
Grant Price per Security (CDN$) |
Number of Securities |
|||||||
| December 2015 |
Stock Options | 3.39 | 65,000 | |||||||
| March 2016 |
Stock Options | 3.96 | 60,000 | |||||||
| March 2016 |
Stock Options | 3.91 | 220,000 | |||||||
| March 2016 |
Stock Options | 3.76 | 40,000 | |||||||
| May 2016 |
Stock Options | 3.66 | 200,000 | |||||||
| June 2016 |
Stock Options | 3.20 | 1,000,000 | |||||||
| July 2016 |
Stock Options | 4.00 | 100,000 | |||||||
| July 2016 |
Stock Options | 3.95 | 40,000 | |||||||
| December 2016 |
Stock Options | 3.65 | 10,000 | |||||||
|
|
|
|||||||||
| Total |
1,735,000 | |||||||||
|
|
|
|||||||||
S-29
We estimate that the net proceeds from the Offering will be approximately US$ million (or US$ million if the Over-Allotment Option is exercised in full) after deducting the Underwriters’ Fee of US$ million (or US$ million if the Over-Allotment Option is exercised in full) and our expenses of the Offering, which are estimated to be US$450,000.
We expect to use the net proceeds of the Offering (without giving effect to the exercise of the Over-Allotment Option) as follows: approximately US$ million for research and development activities including the LN Phase 3 clinical trial activities and approximately US$ million for working capital purposes.
If the Over-Allotment Option is exercised in full, we intend to allocate the additional net proceeds of US$ million to research and development activities including the LN Phase 3 clinical trial activities.
The foregoing discussion does not include any proceeds we may receive from the exercise of Warrants.
Although we intend to use the net proceeds from the Offering as set forth above, there may be circumstances where, for sound business reasons, a reallocation of funds may be deemed prudent or necessary, and may vary materially from that set forth above. Any unallocated funds from the net proceeds of the Offering, if any, may be added to the general working capital of the Company and expended at the discretion of management. See “Risk Factors.”
The actual amount that we spend in connection with each of the intended uses of proceeds may vary significantly from the amounts specified above, and will depend on a number of factors, including those listed under the heading “Risk Factors” in or incorporated by reference in this prospectus supplement.
S-30
If you invest in the Offered Units, your interest will be diluted to the extent of the difference between the public offering price per share of our common shares in this Offering and the net tangible book value per share of our common shares after this Offering. As of September 30, 2016, we had a historical net tangible book value of US$4.2 million, or US$0.11 per common share. Our net tangible book value represents total tangible assets less total liabilities, divided by the number of common shares outstanding on September 30, 2016.
After giving effect to the issue of 11,111,111 Units in this Offering (and assuming no exercise of the Warrants) at a public offering price of US$2.25 per Offered Unit, and after deducting the Underwriters’ Fee and estimated Offering expenses, our as adjusted net tangible book value as of September 30, 2016 would have been US$ million, or US$ per share. This represents an immediate increase in as adjusted net tangible book value of US$ per share to existing shareholders and an immediate dilution of US$ per share to new investors. The following table illustrates this per share dilution:
| Public offering price per Offered Unit (and assuming no exercise of the Warrants) |
US$ | 2.25 | ||||||
| Historical net tangible book value per share as of September 30, 2016 |
US$ | 0.11 | ||||||
| Increase in as adjusted net tangible book value per share attributable to new investors |
US$ | |||||||
|
|
|
|||||||
| As adjusted net tangible book value per share after this Offering |
US$ | |||||||
|
|
|
|||||||
| Dilution per share to new investors participating in this Offering |
US$ | |||||||
|
|
|
If the Underwriters fully exercise the Over-Allotment Option to purchase Additional Offered Units (and assuming no exercise of the Additional Warrants), the adjusted net tangible book value after this Offering would increase to US$ per share, and there would be an immediate dilution of US$ per share to new investors.
The discussion and table above assume (i) the sale by us of 11,111,111 Units, (ii) no exercise of the Underwriters’ Over-Allotment Option, (iii) no exercise of Warrants offered in this Offering, and (iv) no receipt of cash upon the exercise of the Warrants. Upon the exercise of the Warrants, if any, holders of the Warrants will experience additional dilution. The discussion and table above do not take into account further dilution to new investors that could occur upon the exercise of outstanding options and warrants having a per share exercise price less than the Offering Price in this Offering. To the extent that outstanding options and warrants are exercised, new investors will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our shareholders.
The outstanding share information in the table above is based on the number of common shares outstanding as of September 30, 2016 and excludes the following, in each case as of such date:
| • | 4,098,137 common shares issuable upon the exercise of outstanding options outstanding as of September 30, 2016, with a weighted-average exercise price of US$2.84; |
| • | 5,927,223 common shares issuable upon exercise of warrants outstanding as of September 30, 2016 with a weighted average exercise price of US$3.07; |
| • | 951,174 common shares reserved for future issuance under our stock option plans as of September 30, 2016; and |
| • | 1,235,971 common shares issued subsequent to September 30, 2016. |
S-31
DESCRIPTION OF SECURITIES OFFERED UNDER THIS PROSPECTUS SUPPLEMENT
The Offering consists of 11,111,111 Offered Units (1,666,666 Additional Offered Units in the event the Over-Allotment Option is exercised in full). Each Offered Unit will consist of one Unit Share and one-half of one common share purchase warrant, with each Warrant entitling the holder to purchase one Warrant Share at an exercise price of US$3.00, subject to adjustment, at any time until 4:30 p.m. (New York time) on the date that is five years after the closing of the Offering.
Common Shares
We are authorized to issue an unlimited number of common shares, without nominal or par value. As of December 21, 2016, there were 40,030,460 common shares issued and outstanding, 4,078,137 common shares issuable upon exercise of outstanding stock options and 5,004,569 common shares issuable upon exercise of common share purchase warrants.
The holders of common shares are entitled to one vote per share held at meetings of shareholders, to receive such dividends as declared and to receive a share of the remaining property and assets upon dissolution or winding up of Aurinia Pharmaceuticals. The common shares are not subject to any future call or assessment and there are no statutory pre-emptive, conversion or redemption rights attached to such common shares. Provisions as to the modification, amendment or variation of the rights attached to the Common Shares are contained in the Company’s bylaws and the Business Corporations Act (Alberta). Generally speaking, substantive changes to the authorized share structure require the approval of the shareholders of the Company by special resolution (at least two-thirds of the votes cast).
In February 2014, we granted the subscribers in a private placement of our common shares a right of first refusal to participate in any future private placement of common shares or any other class or series of our share capital (or securities convertible into common shares or any other class or series of our share capital), up to their pro rata share of such private placement. This right of first refusal expires upon the earlier of a subscriber no longer holding at least 66 2⁄3% of the common shares purchased in the February 2014 private placement or February 2017.
We have not paid any dividends to date on the common shares. We do not currently expect to pay any dividends on the common shares for the foreseeable future.
Warrants
The Warrants will be issued as individual warrant agreements to the investors. The material terms and provisions of the Warrants offered hereby are summarized below. The following description is subject to, and qualified in its entirety by, the form of Warrant, which we will file on SEDAR under our profile at www.sedar.com and with the SEC as an exhibit to a Report on Form 6-K following closing of this Offering. You should review a copy of the form of Warrant for a complete description of the terms and conditions applicable to the Warrants.
The Unit Shares and the Warrants comprising the Units will separate immediately upon the closing of this Offering. The Warrants are exercisable beginning on the date of issuance, and at any time prior to 4:30 p.m. (New York time) on the date that is five years after the closing of this Offering. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full for the number of common shares purchased upon such exercise (except in the case of a cashless exercise as discussed below). No fractional common shares will be issued in connection with the exercise of a Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price. The holder will not have the right to exercise any portion of the Warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% or 9.99% of the number of our common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Warrants.
S-32
If, at any time during the term of the Warrants, the issuance of Warrant Shares is not covered by an effective registration statement, the holder is permitted to effect a cashless exercise of the Warrants (in whole or in part) by having the holder deliver to us a duly executed exercise notice, canceling a portion of the Warrant in payment of the purchase price payable in respect of the number of common shares purchased upon such exercise.
If we fail to deliver to the investor a certificate representing the Warrant Shares by the third trading day after the exercise date as required by the Warrant, and if the investor purchases common shares after that third trading day to deliver in satisfaction of a sale by the investor of the underlying Warrant Shares that the investor anticipated receiving from us, then, within three trading days of receipt of the investor’s request, we, at the investor’s option, will either (i) pay cash to the investor in an amount equal to the investor’s total purchase price (including brokerage commissions, if any) for the common shares purchased less the exercise price (as described below), or the buy-in price, at which point our obligation to deliver the Warrant (and to issue the underlying Warrant Shares) will terminate, (ii) reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honoured (in which case such exercise shall be deemed rescinded) or (iii) promptly honour our obligation to deliver to the investor a certificate or certificates representing the Warrant Shares and pay cash to the investor in an amount equal to the excess (if any) of the buy-in price over the product of (A) the number of common shares, times (B) the per share closing price of our common shares on the date of the event giving rise to our obligation to deliver the certificate.
Each Warrant represents the right to purchase one common share at an exercise price equal to US$3.00 per share, subject to adjustment. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common shares and also upon any distributions of assets, including cash, stock or other property to our shareholders.
If we consummate any merger, consolidation, sale or other reorganization event in which our common shares are converted into or exchanged for securities, cash or other property, or if we consummate certain sales or other business combinations, then following such event, the holders of the Warrants will be entitled to receive upon exercise of the Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Warrants immediately prior to such event.
Subject to applicable laws, the Warrants may be offered for sale, sold, transferred or assigned without our consent.
There is no public trading market for the Warrants, and we do not expect a market to develop. In addition, we do not intend to apply for listing of the Warrants on any securities exchange or other trading system.
Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of our common shares, the holder of a Warrant does not have the rights or privileges of a holder of our common shares, including any voting rights, until the holder exercises the Warrant.
There is no market through which the Warrants may be sold and purchasers may not be able to resell the Warrants purchased under this prospectus supplement. This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices, the liquidity of such Warrants, and the extent of issuer regulation. See “Risk Factors.”
S-33
Pursuant to the Underwriting Agreement, the Company has agreed to sell and the Underwriters have agreed to purchase on the closing of the Offering, an aggregate of 11,111,111 Offered Units at a price of US$2.25 per Offered Unit, payable against delivery of the Offered Units, subject to the terms and conditions stated in the Underwriting Agreement. In consideration for their services in connection with this Offering, the Underwriters will be paid the Underwriters’ Fee equal to % of the gross proceeds of this Offering ($ per Offered Unit, for an aggregate fee payable by us of $ million, exclusive of the Over-Allotment Option). H.C. Wainwright & Co., LLC will be acting as Lead Underwriter in respect of the Offering. The Offering Price was determined by negotiation between us and the Lead Underwriter on its own behalf and on behalf of the other Underwriter. Subject to the terms and conditions of the Underwriting Agreement, we have agreed to sell to the Underwriters, and each Underwriter has severally agreed to purchase, at the Offering Price less the Underwriting Fee set forth on the cover page of this prospectus supplement, the number of Offered Units listed next to its name in the following table:
| Underwriter |
Number of Offered Units | |||
| H.C. Wainwright & Co., LLC |
8,888,889 | |||
| Cormark Securities Inc. |
2,222,222 | |||
|
|
|
|||
| Total |
11,111,111 | |||
|
|
|
|||
The obligations of the Underwriters under the Underwriting Agreement may be terminated at the discretion of the Lead Underwriter upon the occurrence of certain stated events. The Underwriters are obligated under the Underwriting Agreement to take up and pay for all of the Offered Units if any of the Offered Units are purchased. The Underwriters are offering the Offered Units, subject to prior sale, if, as and when issued to and accepted by it, subject to certain conditions contained in the Underwriting Agreement, such as receipt by the Underwriters of officers’ certificates and legal opinions.
The Company has granted the Underwriters an Over-Allotment Option, exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Underwriters, for a period of 30 days from the date of this prospectus supplement, to purchase up to an additional 1,666,666 of Offered Units at the Offering Price, less the Underwriting Fee, to cover over-allotments, if any, and for market stabilization purposes. The purchase price of one Additional Offered Unit pursuant to the Over-Allotment Option will be equal to the Offering Price. The Over-Allotment Option may be exercisable by the Underwriters in respect of: (i) Additional Offered Units at the Offering Price; or (ii) Additional Shares at a price of US$ per Additional Share; or (iii) Additional Warrants at a price of US$ per Additional Warrant; or (iv) any combination of the Additional Securities, so long as the aggregate number of Additional Shares and Additional Warrants which may be issued under the Over-Allotment Option does not exceed Additional Shares and Additional Warrants. The Additional Securities issued upon exercise of the Over-Allotment Option are qualified for distribution under this short form prospectus. A person who acquires Additional Securities issuable on the exercise of the Over-Allotment Option acquires such Additional Securities under this prospectus supplement regardless of whether the over-allotment position is ultimately filled through the exercise of the Over-Allotment Option or secondary market purchases. If the Over-Allotment Option is exercised in full, the total price to the public, the Underwriters’ Fee and the net proceeds to the Company (before payment of the expenses of the Offering) will be approximately US$28.75 million, US$ million and US$ million, respectively.
The Warrants will be issued as individual warrant agreements to the investors. Each Warrant will entitle the holder thereof to purchase one common share at a price of US$3.00 at any time prior to 4:30 p.m. (New York time) on the date that is five years after the closing of the Offering, after which time the Warrants will expire and be void and of no value. The warrant agreements will contain provisions designed to protect the holders of Warrants against dilution upon the happening of certain events. No fractional common shares will be issued upon the exercise of any Warrants. There is currently no market through which the Warrants may be sold and
S-34
purchasers may not be able to resell such securities. This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices, the liquidity of such securities, and the extent of issuer regulation.
The Offering is being made in the provinces of British Columbia, Alberta and Ontario and in the United States pursuant to the multijurisdictional disclosure system implemented by the SEC and the securities regulatory authorities in Canada. The Offered Units will be offered in Canada by the Underwriters either directly or through their duly registered Canadian broker-dealer affiliates or agents, as applicable. H.C. Wainwright & Co., LLC is not registered as a dealer in the category of investment dealer in any Canadian jurisdiction and, accordingly, will only sell Offered Units into the United States and will not, directly or indirectly, solicit offers to purchase or sell the Offered Units in Canada.
Subscriptions for Offered Units will be received by the Underwriters subject to rejection or allotment in whole or in part and the right is reserved to close the subscription book at any time without notice. It is expected that delivery of the Offered Units offered hereby will be made against payment therefor on or about the closing date, which will be the third business day following the date of this prospectus supplement (this settlement cycle being referred to as “T+3”).
Entities affiliated with the ILJIN SNT Co., Ltd., one of our major stockholders have submitted an indication of interest to purchase up to US$3.0 million of the Offered Units in this Offering. However, because indications of interest are not binding agreements or commitments to purchase, the Underwriters may determine to sell more, fewer or no Offered Units in this Offering to these entities, or these entities may determine to purchase more, fewer or no Offered Units in this Offering.
The Company has agreed in the Underwriting Agreement to reimburse the Underwriters for their legal fees and other expenses in connection with this Offering, in an amount not to exceed US$200,000. The Company has also agreed to indemnify the Underwriters, each of its affiliates and each of its and their directors, officers, partners, employees and agents against certain liabilities and expenses or will contribute to payments that the Underwriters may be required to make in respect thereof.
The Company has also agreed, pursuant to the terms of the Underwriting Agreement that it will not, without the prior written consent of the Lead Underwriter, from the date of execution of the Underwriting Agreement and continuing to and including the date 45 days after the date of this prospectus supplement (the “Lock-Up Period”), (A) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, any common shares or any securities convertible into or exercisable or exchangeable for common shares or (B) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common shares, whether any such transaction described in clause (A) or (B) above is to be settled by delivery of common shares or such other securities, in cash or otherwise, except for (1) sales of the Offered Units to the Underwriters pursuant to the Underwriting Agreement, (2) the issuance of Warrant Shares on the exercise of Warrants, (3) grants of options or the issuance of common shares by the Company pursuant to equity incentive plans, or (4) issuance of shares upon exercise or conversion of securities outstanding as of the date thereof. The Company further agreed pursuant to the Underwriting Agreement not to accelerate the vesting of any option or warrant or the lapse of any repurchase right prior to the expiration of the Lock-Up Period except immediately prior to the closing of a transaction that would involve the sale of all of the common shares of the Company to a third party.
As a condition precedent to the Underwriters’ obligation to close the Offering, subject to customary exemptions permitting dispositions to trusts for the direct or indirect benefit of the director or officer and/or the immediate family of such person, tenders to a take-over bid or acquisition transaction and pursuant to any existing 10b5-1 plans, all directors and officers of the Company shall be required to execute and deliver written undertakings in favour of the Underwriters agreeing not to sell, transfer, pledge (other than as disclosed to the Underwriters in
S-35
writing), assign, or otherwise dispose of any securities of the Company owned, directly or indirectly by such directors or officers, until 45 days following the date of this prospectus supplement, without the prior written consent of the Lead Underwriter on behalf of the Underwriters.
The Underwriters propose to offer the Offered Units initially at the public Offering Price on the cover page of this prospectus supplement. After the Underwriters have made a reasonable effort to sell all of the Offered Units offered by this prospectus supplement at the Offering Price specified herein, the Offering Price may be decreased, and further changed from time to time and the compensation realized by the Underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the Offered Units is less than the gross proceeds paid by the Underwriters to the Company. Any such reduction will not affect the proceeds received by the Company.
In order to facilitate the Offering, the Underwriters may engage in transactions that stabilize, maintain or otherwise affect the market price of the common shares in accordance with Regulation M under the Exchange Act.
The Underwriters may over-allot common shares in connection with the Offering, thus creating a short position for its own account. Short sales involve the sale by the Underwriters of a greater number of shares than they are committed to purchase in the Offering. To cover these short sales positions or to stabilize the market price of the common shares, the Underwriters may bid for, and purchase, common shares in the open market. These transactions may be effected on the TSX, the NASDAQ or otherwise. Additionally, the representatives, on behalf of the Underwriters, may also reclaim selling concessions allowed to another underwriter or dealer. Similar to other purchase transactions, the Underwriters’ purchases to cover the syndicate short sales or to stabilize the market price of the common shares may have the effect of raising or maintaining the market price of the common shares or preventing or mitigating a decline in the market price of the common shares. As a result, the price of the common shares may be higher than the price that might otherwise exist in the open market. No representation is made as to the magnitude or effect of any such stabilization or other activities. The Underwriters are not required to engage in these activities and, if commenced, may discontinue any of these activities at any time.
Pursuant to rules and policy statements of certain Canadian securities regulators, the Underwriters may not, at any time during the period ending on the date the selling process for the Offered Units ends and all stabilization arrangements relating to the Offered Units are terminated, bid for or purchase common shares. The foregoing restrictions are subject to certain exceptions including (a) a bid for or purchase of common shares if the bid or purchase is made through the facilities of the TSX, in accordance with the Universal Market Integrity Rules of Market Regulation Services Inc., (b) a bid or purchase on behalf of a client, other than certain prescribed clients, provided that the client’s order was not solicited by the Underwriters, or if the client’s order was solicited, the solicitation occurred before the commencement of a prescribed restricted period, and (c) a bid or purchase to cover a short position entered into prior to the commencement of a prescribed restricted period. The Underwriters may engage in market stabilization or market balancing activities on the TSX where the bid for or purchase of the common shares is for the purpose of maintaining a fair and orderly market in the common shares, subject to price limitations applicable to such bids or purchases. Such transactions, if commenced, may be discontinued at any time.
The Company has applied to list the Unit Shares and the Warrant Shares on the TSX. Listing will be subject to the Company fulfilling all of the requirements of the TSX. The Company has also applied to list the Unit Shares and the Warrant Shares on the NASDAQ. Listing will be subject to the Company fulfilling all of the requirements of the NASDAQ.
This prospectus supplement and the accompanying prospectus in electronic format may be made available on the websites maintained by one or more of the Underwriters or their U.S. affiliates participating in this Offering. The Underwriters may agree to allocate a number of Offered Units to the Underwriters and their U.S. affiliates for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to the
S-36
Underwriters and their U.S. affiliates that may make Internet distributions on the same basis as other allocations. Other than the prospectus supplement and the accompanying prospectus in electronic format, the information on these websites is not part of this prospectus supplement or the registration statement of which this prospectus supplement forms a part, has not been approved or endorsed by us or any Underwriter in its capacity as underwriter, and should not be relied upon by investors.
Certain of the Underwriters and their affiliates have provided in the past to us and our affiliates, and may provide from time to time in the future, certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the Underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
This prospectus supplement also covers the distribution of the Warrant Shares by the Company.
S-37
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
In this summary, an otherwise undefined term that first appears in quotation marks has the meaning ascribed to it in the Income Tax Act (Canada) (the “Tax Act”).
In the opinion of Borden Ladner Gervais LLP, Canadian legal counsel to Aurinia Pharmaceuticals, and Stikeman Elliott LLP, Canadian legal counsel to the Underwriters, the following fairly summarizes the principal Canadian federal income tax considerations under the Tax Act generally applicable as of this date to an investor who acquires Offered Units pursuant to the Offering and who, at all relevant times for the purposes of the Tax Act,
| • | deals at arm’s length with Aurinia Pharmaceuticals and the Underwriters, |
| • | is not affiliated with Aurinia Pharmaceuticals or the Underwriters, |
| • | holds all Unit Shares and Warrants, and will hold all Warrant Shares acquired on exercise of a Warrant as capital property, |
and is not
| • | exempt from tax under Part I of the Tax Act, |
| • | a “financial institution” for the purposes of the “mark-to-market” property rules in the Tax Act, |
| • | a “specified financial institution”, |
| • | an entity or partnership an interest in which is a “tax shelter investment”, |
| • | a taxpayer who reports its “Canadian tax results” in a currency other than Canadian currency, or |
| • | a taxpayer that has or will enter into a “derivative forward agreement” with respect to Unit Shares Warrant Shares or Warrants, |
(each such investor, in this summary, a “Holder”).
In this summary, unless the context otherwise requires, a reference to a Share means a Unit Share or a Warrant Share as the case may be.
A Holder’s Shares or Warrants, as applicable, will generally be considered to be capital property of the Holder provided that the Holder does not use the Shares or Warrants in the course of carrying on a business of trading or dealing in securities, and has not acquired or been deemed to have acquired the Shares or Warrants in one or more transactions considered to be an adventure or concern in the nature of trade. A Holder who is resident in Canada and whose Shares might not otherwise be capital property may, subject to certain restrictions and limitations in the Tax Act, be entitled to elect irrevocably pursuant to subsection 39(4) of the Tax Act that the Holder’s Shares, and every other “Canadian security” of the Holder, be capital property. Any Holder who is considering making a subsection 39(4) election should consult the Holder’s Canadian tax advisers before making the election. A subsection 39(4) election will not apply to, or to a disposition of, Warrants.
This summary is based on the current provisions of the Tax Act and the Income Tax Regulations (Canada) (the “Regulations”) in force as of the date hereof, all specific proposals to amend the Tax Act or Regulations publicly announced by or on behalf of the Minister of Finance of Canada on or before the date hereof, and counsel’s understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”). It is assumed that all such amendments will be enacted as currently proposed and that there will be no other change to the Tax Act, the Regulations, or the CRA’s administrative policies and assessing practices, although no assurance can be given in these respects. This summary does not otherwise take into account or anticipate any change in law or administrative policy or assessing practice whether by legislative, governmental, or judicial decision or action, and does not take into account or consider any provincial, territorial or foreign income tax considerations, which may differ significantly from the Canadian federal income tax considerations discussed below.
S-38
Additional considerations, not discussed in this summary, may be applicable to a Holder that is a corporation resident in Canada, and is, or becomes, or does not deal at arm’s length for purposes of the Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or event or series of transactions or events that includes the acquisition of the Offered Units, controlled by a non-resident corporation for purposes of the “foreign affiliate dumping” rules in section 212.3 of the Tax Act. Such Holders should consult their Canadian tax advisers with respect to the consequences of acquiring Offered Units.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, and should not be construed to be, legal or tax advice to any particular Holder. Each Holder should consult the Holder’s own tax advisers with respect to the tax and legal consequences of acquiring Offered Units, and the holding and disposition of Unit Shares, Warrants or Warrant Shares, applicable to the Holder’s particular circumstances.
Currency Conversion
Subject to certain exceptions that are not discussed in this summary, all amounts relevant to computing a Holder’s liability for tax (including dividends, adjusted cost base, and proceeds of disposition) under the Tax Act must, for the purposes of the Tax Act, be determined in Canadian dollars based on the daily noon rate quoted by the Bank of Canada for the applicable day (or, if such day is after March 1, 2017, the single rate quoted by the Bank of Canada for the applicable day) or such other rate of exchange that is acceptable to the CRA. The amount of any dividend required to be included in a Holder’s income, or of any capital gain or capital loss realized by a Holder, may be affected by fluctuations in the Canadian dollar against other currencies.
Allocation of Cost and Adjusted Cost Base
Each Holder must allocate the Offering Price of an Offered Unit on a reasonable basis between the Unit Share and the one-half of one Warrant included in the Unit to determine the cost of each to the Holder for purposes of the Tax Act. The Company intends to allocate US$2.24 of the Offering Price to the Unit Share and US$0.01 of the Offering Price to the one-half of one Warrant included in an Offered Unit. Although the Company believes this allocation to be reasonable, it is not binding on the CRA or the Holder.
A Holder’s initial adjusted cost base of the Unit Share included in an Offered Unit will be determined by averaging the cost of the Unit Share with the adjusted cost base to the Holder of all Shares owned by the Holder as capital property immediately before such acquisition.
Similarly, a Holder’s initial adjusted cost base of each whole Warrant acquired pursuant to the Offering initially will be the average cost of all Warrants so acquired by the Holder.
Resident Holders
The following section of this summary applies solely to Holders each of whom at all relevant times is or is deemed to be resident solely in Canada for the purposes of the Tax Act (each a “Resident Holder”).
Exercise or Expiry of Warrants
A Resident Holder who exercises a Warrant in a taxation year and thereby acquires a Warrant Share will not thereby realize any capital gain or capital loss. The Holder’s cost of the Warrant Share will equal the Holder’s adjusted cost base of the Warrant plus the exercise price paid for the Warrant Share. The Holder’s adjusted cost base of the Warrant Share so acquired will be determined by averaging its cost with the Holder’s adjusted cost base of all of the Holder’s Shares determined immediately before the acquisition.
S-39
A Resident Holder of a Warrant that expires before exercise will generally realize a capital loss equal to the Resident Holder’s adjusted cost base of the Warrant. The tax treatment of capital gains and capital losses is discussed below under the subheading “Capital Gains and Capital Losses”.
Dividends
A Resident Holder who is an individual (other than certain trusts) and receives or is deemed to receive a dividend on the holder’s Shares in a taxation year will generally be required to include the amount of the dividend in income for the taxation year, subject to the gross-up and dividend tax credit rules applicable to a “taxable dividend” received from a “taxable Canadian corporation”, including the enhanced gross-up and dividend tax credit rules applicable to any dividend that Aurinia Pharmaceuticals designates as an “eligible dividend” in accordance with the Tax Act.
A Resident Holder that is a corporation will generally be required to include the amount of any such dividend in its income for the taxation year, and entitled to deduct an equivalent amount from its taxable income for the year. In certain circumstances, subsection 55(2) of the Tax Act may deem some or all of the dividend to be a gain from the disposition of capital property rather than a dividend, in which case the rules described below under “Capital Gains and Losses” would apply. Corporate Resident Holders should consult their own tax advisers regarding the potential application of subsection 55(2) to their particular circumstances.
A Resident Holder that is a “private corporation” or “subject corporation” may be subject to a refundable tax under Part IV of the Tax Act to the extent that the dividend is deductible in computing the corporation’s taxable income. This refundable tax generally will be refunded to the corporate Resident Holder if it pays sufficient taxable dividends while it is a private corporation.
Disposition of Shares or Warrants
A Resident Holder who disposes or is deemed to dispose of a Warrant (other than on the exercise or expiry thereof) or Share in a taxation year will generally realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of the Share or Warrant, as applicable, net of any reasonable costs of disposition, are greater (or less) than adjusted cost base of the security to the Resident Holder. The tax treatment of capital gains and capital losses is discussed in greater detail below under the subheading “Capital Gains and Capital Losses.”
Capital Gains and Capital Losses
A Resident Holder who realizes or is deemed to realize a capital gain or capital loss in a taxation on the disposition of a Share or Warrant will generally be required to include one half of any such capital gain (a “taxable capital gain”) in income for the year, and entitled to deduct one half of any such capital loss (an “allowable capital loss”) from taxable capital gains realized by the Resident Holder in the year or, to the extent not so deductible, in any of the Holder’s three preceding taxation years or any subsequent taxation year, subject to the detailed rules in the Tax Act regarding the deductibility of allowable capital losses.
The amount of any capital loss realized on the disposition or deemed disposition of a Share by a Resident Holder that is a corporation may be reduced by the amount of dividends that the Resident Holder received or is deemed to have received on the Share or a share substituted therefor, to the extent and in the circumstances specified by the Tax Act. Similar rules may apply to a Share owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary, as the case may be. Resident Holders to whom these rules may be relevant should consult their own tax advisers.
A Resident Holder that is throughout the relevant taxation year a “Canadian-controlled private corporation” may be liable to pay an additional refundable tax on certain investment income including taxable capital gains. This refundable tax generally will be refunded to the corporate Resident Holder if it pays sufficient taxable dividends while it is a private corporation.
S-40
Minimum Tax
A Resident Holder who is an individual (including certain trusts) and realizes a capital gain or receives a dividend may thereby be subject to minimum tax under the Tax Act. Such Resident Holders should consult their own tax advisers in this regard.
Non-resident Holders
The following section of this summary is applicable solely to Holders each of whom, at all relevant times for the purposes of the Tax Act,
| • | is not resident in Canada, |
| • | does not use or hold, and is not deemed to use or hold, Shares or Warrants in connection with carrying on a business in Canada, |
| • | is not an “authorized foreign bank”, and |
| • | is not an insurer that carries on business in Canada and elsewhere, |
(each a “Non-resident Holder”).
Disposition of Shares or Warrants
A Non-resident Holder who disposes or is deemed to dispose of a Share or Warrant generally will not be subject to tax under the Tax Act in respect of any capital gain, or entitled to deduct any capital loss, thereby realized unless the Share or Warrant, at the time of the disposition,
| • | is “taxable Canadian property”, and |
| • | is not “treaty-protected property”, |
of the Non-resident Holder.
Generally, a Non-resident Holder’s Share or Warrant, as applicable, should not be taxable Canadian property to the Non-resident Holder at the time of disposition if at that time the Shares are listed on a designated stock exchange (which currently includes the TSX and NASDAQ) unless, at the time of disposition or at any time in the preceding 60 months,
| • | the Non-resident Holder, one or more persons with whom the Non-resident Holder did not deal at arm’s length for the purposes of the Tax Act, or one or more partnerships in which the Non-resident Holder or persons with whom the Non-resident Holder did not deal at arm’s length holds or held a membership interest (either directly or indirectly through one or more partnerships), alone or in any combination owned 25% or more of the issued shares of any class or series of shares of Aurinia Pharmaceuticals, and |
| • | the Share derived more than 50% of its fair market value directly or indirectly from, or from any combination of, real or immovable property situated in Canada, “Canadian resource properties”, “timber resource properties”, or options in respect of, interests in, or for civil law purposes rights in, any such property, whether or not the property exists, |
or the Share was otherwise deemed by a provision of the Tax Act to be taxable Canadian property at the time of disposition.
Generally, a Non-resident Holder’s Shares or Warrants will be treaty-protected property at the time of disposition if, at that time, the terms of a tax treaty between Canada and another country exempt the Non-resident Holder from tax under Part I of the Tax Act on any gain from the disposition of the Shares or Warrants, as applicable.
S-41
Non-resident Holders should consult their own tax advisers regarding whether their Shares or Warrants are taxable Canadian property or treaty-protected property.
A Non-resident Holder who disposes or is deemed to dispose of a Share or Warrant in a taxation year at a time when the Share or Warrant is taxable Canadian property and is not treaty–protected property of the Holder generally will be required to file a Canadian tax return to report the disposition. The Non-resident Holder generally will be required to include any resulting taxable capital gain in the Non-resident Holder’s taxable income earned in Canada for the taxation year, and entitled to deduct any resulting allowable capital loss from taxable capital gains included in the Non-resident Holder’s taxable income earned in Canada for the year or, to the extent not so deductible, in any of the Holder’s three preceding taxation years or any subsequent taxation year, subject to the detailed rules regarding the deductibility of allowable capital losses in the Tax Act.
Dividends
A Non-resident Holder to whom a dividend is or is deemed to be paid or credited on the Non-resident Holder’s Shares will generally be subject to Canadian withholding tax equal to 25% of the gross amount of the dividend, or such lower rate as may be provided by an applicable income tax treaty between Canada and another country. The rate of withholding tax under the Canada-U.S. Income Tax Convention (1980) (the “U.S. Treaty”) applicable to a dividend paid or credited to a Non-resident Holder who beneficially owns the dividend, and is a resident of the United States under the U.S. Treaty and entitled to its benefits, generally is 15%.
S-42
MATERIAL U.S. FEDERAL INCOME TAXATION CONSIDERATIONS
The following discussion describes the material U.S. federal income tax consequences to U.S. Holders (as defined below) relating to the acquisition, ownership and disposition of Units acquired pursuant to this Offering, the acquisition, ownership and disposition of the Unit Shares acquired as part of the Units, the exercise, disposition and lapse of Warrants acquired as part of the Units, and the acquisition, ownership and disposition of Warrant Shares received upon exercise of the Warrants. This discussion applies to U.S. Holders that purchase Units pursuant to the Offering and hold such Units, Unit Shares, Warrants and Warrant Shares as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”). This discussion is based on the Code, U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect. This discussion does not address all of the U.S. federal income tax consequences that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally mark their securities to market for U.S. federal income tax purposes, tax-exempt entities, retirement plans, regulated investment companies, real estate investment trusts, certain former citizens or residents of the United States, persons who hold Units, Unit Shares, Warrants, or Warrant Shares as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons who received their Units as compensatory payments, persons that have a “functional currency” other than the U.S. dollar, persons that own directly, indirectly or through attribution 10% or more of the voting power of our shares, corporations that accumulate earnings to avoid U.S. federal income tax, partnerships and other pass-through entities, and investors in such pass-through entities). This discussion does not address any U.S. state or local or non-U.S. tax consequences or any U.S. federal estate, gift or alternative minimum tax consequences.
As used in this discussion, the term “U.S. Holder” means a beneficial owner of Units, Unit Shares, Warrants, or Warrant Shares that is, for U.S. federal income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial decisions or (y) that has elected under applicable U.S. Treasury regulations to be treated as a domestic trust for U.S. federal income tax purposes.
If an entity treated as a partnership for U.S. federal income tax purposes holds Units, Unit Shares, Warrants, or Warrant Shares, the U.S. federal income tax consequences relating to an investment in the Units, Unit Shares, Warrants, or Warrant Shares will depend in part upon the status and activities of such entity and the particular partner. Any such entity should consult its own tax advisor regarding the U.S. federal income tax consequences applicable to it and its partners of the purchase, ownership and disposition of Units, Unit Shares, Warrants and Warrant Shares.
Persons considering an investment in Units should consult their own tax advisors as to the particular tax consequences applicable to them relating to the purchase, ownership and disposition of Units, Unit Shares, Warrants and Warrant Shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Acquisition of Units
There is no authority directly addressing the treatment, for U.S. federal income tax purposes, of securities with terms substantially the same as the Units, and, therefore, such treatment is not entirely clear. We intend to treat the acquisition by a U.S. Holder of a Unit as the acquisition of one Unit Share and one-half of one Warrant for U.S. federal income tax purposes. The purchase price for each Unit will be allocated between these two
S-43
components in proportion to their relative fair market values at the time the Unit is purchased by the U.S. Holder. This allocation of the purchase price for each Unit will establish a U.S. Holder’s initial tax basis for U.S. federal income tax purposes in the Unit Share and one-half of one Warrant that comprise each Unit. For this purpose, we will allocate US$2.24 of the purchase price for the Unit to the Unit Share and US$0.01 of the purchase price for each Unit to the one-half of one Warrant.
Our view of the characterization of the Units, and the purchase price allocation, are not, however, binding on the IRS or the courts. Because there are no authorities that directly address instruments that are similar to the Units, no assurance can be given that the IRS or the courts will agree with the characterization described above or the discussion below. Accordingly, prospective investors are urged to consult their own tax advisors regarding the U.S. federal tax consequences of an investment in a Unit (including alternative characterizations of a Unit) and with respect to any tax consequences arising under the laws of any state, local or non-U.S. taxing jurisdiction. Unless otherwise stated, the following discussions are based on the assumption that the characterization of the Units and the allocation described above are accepted for U.S. federal income tax purposes.
Passive Foreign Investment Company Consequences
In general, a corporation organized outside the United States will be treated as a passive foreign investment company, (“PFIC”), for any taxable year in which either (1) at least 75% of its gross income is “passive income”, or the “PFIC income test”, or (2) on average at least 50% of its assets, determined on a quarterly basis, are assets that produce passive income or are held for the production of passive income (the “PFIC asset test”). Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income generally include cash, even if held as working capital or raised in a public offering, marketable securities, and other assets that may produce passive income. Generally, in determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
We do not believe we were a PFIC for the year ended December 31, 2015. While we also do not believe we will be a PFIC for the current taxable year, because PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, there can be no assurance that we will not be a PFIC for the current or future taxable years. Because we may hold a substantial amount of cash and cash equivalents following this offering, and because the calculation of the value of our assets may be based in part on the value of common shares, which may fluctuate considerably, we may also be a PFIC in future taxable years under the PFIC asset test. Even if we determine that we are not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not successfully challenge our position. Our status as a PFIC is a fact-intensive determination made on an annual basis. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status and also expresses no opinion with regard to our expectations regarding our PFIC status.
If we are a PFIC in any taxable year during which a U.S. Holder owns Unit Shares, Warrants or Warrant Shares, the U.S. Holder could be liable for additional taxes and interest charges under the “PFIC excess distribution regime” upon (1) a distribution paid during a taxable year that is greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the Unit Shares, Warrants or Warrant Shares, and (2) any gain recognized on a sale, exchange or other disposition, including a pledge, of the Unit Shares, Warrants or Warrant Shares, whether or not we continue to be a PFIC. Under the PFIC excess distribution regime, the tax on such distribution or gain would be determined by allocating the distribution or gain ratably over the U.S. Holder’s holding period for Unit Shares, Warrants or Warrant Shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the current taxable year. The amount allocated to other taxable years will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable, to ordinary income for each
S-44
such taxable year, and an interest charge, generally applicable to underpayments of tax, will be added to the tax. Under proposed Treasury Regulations that are prospectively effective until finalized, if a U.S. Holder has an option, warrant, or other right to acquire stock of a PFIC (such as the Warrants), such option, warrant or right is considered to be PFIC stock subject to the PFIC excess distribution regime. Under the proposed Treasury Regulations, a U.S. Holder’s holding period for the Warrant Shares will begin on the date the U.S. Holder acquires the Units. This will adversely impact the availability of the QEF Election (defined below) and “mark-to-market” election (described below) with respect to the Warrant Shares. Thus, a U.S. Holder may have to account for Warrant Shares and Unit Shares under the PFIC rules and the applicable elections differently.
If we are a PFIC for any year during which a U.S. Holder holds Unit Shares, Warrants or Warrant Shares, we must generally continue to be treated as a PFIC by that holder for all succeeding years during which the U.S. Holder holds the Unit Shares, Warrants or Warrant Shares, unless we cease to meet the requirements for PFIC status and the U.S. Holder makes a “deemed sale” election with respect to the Unit Shares, Warrants or Warrant Shares. If the election is made, the U.S. Holder will be deemed to sell the Unit Shares, Warrants or Warrant Shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime. After the deemed sale election, the U.S. Holder’s Unit Shares, Warrants or Warrant Shares would not be treated as shares of a PFIC unless we subsequently become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds Unit Shares, Warrants or Warrant Shares and one of our non-U.S. corporate subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions. Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries.
If we are a PFIC, a U.S. Holder will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on Unit Shares or Warrant Shares if such U.S. Holder makes a valid “mark-to-market” election for such shares. A mark-to-market election is available to a U.S. Holder only for “marketable stock.” Our common shares will be marketable stock as long as they remain listed on the NASDAQ and are regularly traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. If a mark-to-market election is in effect, a U.S. Holder generally would take into account, as ordinary income each year, the excess of the fair market value of common shares held at the end of such taxable year over the adjusted tax basis of such common shares. The U.S. Holder would also take into account, as an ordinary loss each year, the excess of the adjusted tax basis of such common shares over their fair market value at the end of the taxable year, but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the mark-to-market election. The U.S. Holder’s tax basis in common shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. Any gain from a sale, exchange or other disposition of common shares in any taxable year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated first as ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as capital loss.
A mark-to-market election will not apply to common shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any non-U.S. subsidiaries that we may organize or acquire in the future. Accordingly, a U.S. Holder may continue to be subject to tax under the PFIC excess distribution regime with respect to any lower-tier PFICs that we may organize or acquire in the future notwithstanding the U.S. Holder’s mark-to-market election for the common shares.
Any mark-to-market election made by a U.S. Holder for the Unit Shares will also apply to such U.S. Holder’s Warrant Shares. As a result, if a mark-to-market election has been made by a U.S. Holder with respect to Unit
S-45
Shares, any Warrant Shares received will automatically be marked-to-market in the year of exercise of the Warrant. Because, under the proposed Treasury Regulations referenced above, a U.S. Holder’s holding period for Warrant Shares includes the period during which such U.S. Holder held the Warrants, a U.S. Holder will be treated as making a mark-to-market election with respect to its Warrant Shares after the exercise of the Warrant unless the Warrant Shares are acquired in the same tax year as the year in which the U.S. Holder acquired its Units. Consequently, the excess distribution regime provisions described above generally will apply to the mark-to market gain realized in the tax year in which Warrant Shares are received. However, the general mark-to-market rules will apply to subsequent tax years.
The tax consequences that would apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing fund (“QEF”) election. At this time we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make a QEF election, prospective investors should assume that a QEF election will not be available.
Each U.S. person that is an investor of a PFIC is generally required to file an annual information return on IRS Form 8621 containing such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
The U.S. federal income tax rules relating to PFICs are very complex. Prospective U.S. investors are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of Units, Unit Shares, Warrant Shares and Warrants, the consequences to them of an investment in a PFIC, any elections available with respect to the common shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of Units, Unit Shares, Warrant Shares and Warrants of a PFIC.
Exercise and Disposition of Warrants
The following discussion is subject in its entirety to the rules described above under the heading “—Passive Foreign Investment Company Consequences.”
Exercise of Warrants
A U.S. Holder will not recognize gain or loss on the exercise of a Warrant and related receipt of a Warrant Share (unless, and to the extent that, cash is received in lieu of the issuance of a fractional Warrant Share). A U.S. Holder’s initial tax basis in the Warrant Share received on the exercise of a Warrant will be equal to the sum of (a) such U.S. Holder’s tax basis in such Warrant plus (b) the exercise price paid by such U.S. Holder on the exercise of such Warrant. If we are a PFIC, a U.S. Holder’s holding period for the Warrant Share received on the exercise of a Warrant should begin on the date on which such U.S. Holder acquired its Warrants. If we are not a PFIC with respect to a U.S. Holder, the holding period for the Warrant Shares received by the U.S. Holder will begin on the date following the date of exercise and will not include the period during which the U.S. Holder held its Warrants.
In certain limited circumstances, a U.S. Holder may be permitted to undertake a cashless exercise of Warrants into Warrant Shares. The U.S. federal income tax treatment of a cashless exercise of Warrants into Warrant Shares is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a Warrant described in the preceding paragraph. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of Warrants.
Disposition of Warrants
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of a Warrant in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and
S-46
(b) such U.S. Holder’s tax basis in the Warrant sold or otherwise disposed of. Subject to the PFIC rules discussed above, any such gain or loss generally will be a capital gain or loss, which will be long term capital gain or loss if the Warrant is held for more than one year. Deductions for capital losses are subject to complex limitations under the Code.
Expiration of Warrants Without Exercise
Upon the lapse or expiration of a Warrant, a U.S. Holder will recognize a loss in an amount equal to such U.S. Holder’s tax basis in the Warrant. Any such loss generally will be a capital loss and will be long-term capital loss if the Warrants are held for more than one year. Deductions for capital losses are subject to complex limitations under the Code.
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of Warrant Shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of the Warrants, may be treated as a constructive distribution to a U.S. Holder of the Warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. Holder’s proportionate interest in our “earnings and profits” or our assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to the shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. (See more detailed discussion of the rules applicable to distributions made by us at “— Distributions on Unit Shares or Warrant Shares” below).
Distributions on Unit Shares or Warrant Shares
Subject to the discussion above under “— Passive Foreign Investment Company Consequences,” a U.S. Holder that receives a distribution with respect to Unit Shares or Warrant Shares generally will be required to include the gross amount of such distribution in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s Unit Shares or Warrant Shares. To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s Unit Shares or Warrant Shares, the remainder will be taxed as capital gain. Because we may not account for our earnings and profits in accordance with U.S. federal income tax principles, U.S. Holders should expect all distributions to be reported to them as dividends. Distributions on Unit Shares or Warrant Shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute passive category income. Such dividends will not be eligible for the “dividends received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations.
Dividends paid by a “qualified foreign corporation” are eligible for taxation at a reduced capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that certain requirements are met. However, if we are a PFIC for the taxable year in which the dividend is paid or the preceding taxable year (see discussion above under “— Passive Foreign Investment Company Consequences”), we will not be treated as a qualified foreign corporation, and therefore the reduced capital gains tax rate described above will not apply. Each U.S. Holder is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular circumstances.
S-47
A non-United States corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on common shares that are readily tradable on an established securities market in the United States. We believe that we qualify as a resident of Canada for purposes of, and are eligible for the benefits of, the U.S.-Canada Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Canada Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange of information provision. Therefore, subject to the discussion above under “— Passive Foreign Investment Company Consequences,” if the U.S.-Canada Treaty is applicable, such dividends will generally be “qualified dividend income” in the hands of individual U.S. Holders, provided that certain conditions are met, including holding period and the absence of certain risk reduction transaction requirements are met.
Sale, Exchange or Other Disposition of Unit Shares or Warrant Shares
Subject to the discussion above under “— Passive Foreign Investment Company Consequences,” a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of Unit Shares or Warrant Shares in an amount equal to the difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received) on the sale, exchange or other disposition and such U.S. Holder’s adjusted tax basis in the Unit Shares or Warrant Shares. Such capital gain or loss generally will be long-term capital gain taxable at a reduced rate for non-corporate U.S. Holders or long-term capital loss if, on the date of sale, exchange or other disposition, the Unit Shares or Warrant Shares were held by the U.S. Holder for more than one year. Any capital gain of a non-corporate U.S. Holder that is not long-term capital gain is taxed at ordinary income rates. The deductibility of capital losses is subject to limitations. Any gain or loss recognized from the sale or other disposition of Unit Shares or Warrant Shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of Units, Warrants, Unit Shares or Warrant Shares. If you are a United States person that is an individual, estate or trust, you are encouraged to consult your tax advisors regarding the applicability of this Medicare tax to your income and gains in respect of your investment in Units, Warrants, Unit Shares or Warrant Shares.
Information Reporting and Backup Withholding
U.S. Holders may be required to file certain U.S. information reporting returns with the IRS with respect to an investment in Units, Warrants, Unit Shares or Warrant Shares, including, among others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment Company Consequences”, each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information. U.S. Holders paying more than US$100,000 for Units may be required to file IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to comply with the required information reporting.
Dividends on and proceeds from the sale or other disposition of Units, Warrants, Unit Shares or Warrant Shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if the holder (1) fails to provide an accurate United States taxpayer identification number or otherwise establish a basis for exemption, or (2) is described in certain other categories of persons. However, U.S. Holders that are corporations generally are excluded from these information reporting
S-48
and backup withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
U.S. Holders should consult their own tax advisors regarding the backup withholding tax and information reporting rules.
EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN UNITS, WARRANTS, UNIT SHARES OR WARRANT SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
S-49
Charles A. Rowland, Jr., the President and Chief Executive Officer and a director of Aurinia Pharmaceuticals Inc., and Gregory Ayers, Hyuek Joon Lee, David Jayne and Lorin Jeffry Randall, each a director of Aurinia Pharmaceuticals Inc., reside outside of Canada and have appointed an agent for service of process in Canada, as set out in the table below.
| Name of Person |
Name and Address of Agent | |
| Charles A. Rowland, Jr., Gregory Ayers, Hyuek Joon Lee, David Jayne and Lorin Jeffry Randall |
Borden Ladner Gervais LLP 1200 Waterfront Centre 200 Burrard Street, P.O. Box 48600 Vancouver, British Columbia, Canada V7X 1T2 Attention: Stephen P. Robertson |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-10 under the U.S. Securities Act of 1933, as amended, relating to the offering of our securities, of which this prospectus supplement forms a part. This prospectus supplement does not contain all of the information set forth in the registration statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC. Reference is made to such registration statement and the exhibits thereto for further information with respect to us and the common shares.
We are required to file with the various securities commissions or similar authorities in each of the applicable provinces and territories of Canada, annual and quarterly reports, material change reports and other information. We are also an SEC registrant subject to the informational requirements of the U.S. Securities Exchange Act of 1934, as amended, and, accordingly, file with, or furnish to, the SEC certain reports and other information. Under the multi-jurisdictional disclosure system adopted by the United States and Canada, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ from those in the United States. You may read and copy any document we file with or furnish to the SEC at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the same documents from the public reference room by paying a fee. Please call the SEC at 1-800-SEC-0330 or contact them at www.sec.gov for further information on the public reference room and copying charges.
S-50
ENFORCEABILITY OF CERTAIN CIVIL LIABILITIES
We are a corporation incorporated and existing under the laws of the Province of Alberta. Most of our officers and directors and some of the experts named in this prospectus supplement are not residents of the United States, and many of our assets and all or a substantial portion of assets of such persons are located outside of the United States. We have appointed CT Corporation System as our agent for service of process in the United States. We have also filed with the SEC, concurrently with the registration statement on Form F-10 relating to the accompanying prospectus, an appointment of agent for service of process on Form F-X. Under the Form F-X, we appointed CT Corporation System as our agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC and any civil suit or action brought against or involving us in a United States court arising out of or related to or concerning the Offering.
However, it may be difficult for United States investors to effect service of process within the United States upon those officers or directors who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon Aurinia Pharmaceuticals’ civil liability and the civil liability of such officers or directors under United States federal securities laws or the securities or “blue sky” laws of any state within the United States. We have been advised by our Canadian counsel, Borden Ladner Gervais LLP, that, subject to certain limitations, a judgment of a United States court predicated solely upon civil liability under United States federal securities laws may be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. Aurinia Pharmaceuticals has also been advised by Borden Ladner Gervais LLP, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of liability predicated solely upon United States federal securities laws.
S-51
Certain legal matters in connection with the Offering will be passed upon on our behalf by Borden Ladner Gervais LLP with respect to Canadian legal matters and by Cooley LLP, Palo Alto, California with respect to U.S. legal matters and on behalf of the Underwriters by Stikeman Elliott LLP with respect to Canadian legal matters and by Goodwin Procter LLP, with respect to U.S. legal matters.
The partners and associates of Borden Ladner Gervais LLP (Canadian counsel for Aurinia Pharmaceuticals), as a group, beneficially own, directly or indirectly, less than one percent of the outstanding securities of Aurinia Pharmaceuticals. The partners and associates of Stikeman Elliott LLP (Canadian counsel for the Underwriters), as a group, beneficially own, directly or indirectly, less than one percent of the outstanding securities of Aurinia Pharmaceuticals.
PricewaterhouseCoopers LLP, our auditor, issued an auditor’s report dated March 18, 2016 in respect of our consolidated financial statements, which comprise the consolidated statements of financial position as at December 31, 2015 and December 31, 2014, and the consolidated statements of operations and comprehensive loss, consolidated statements of changes in shareholders’ equity (deficit) and cash flows for the years ended December 31, 2015 and December 31, 2014, and the related notes. PricewaterhouseCoopers LLP has advised us that they are independent with respect to Aurinia Pharmaceuticals Inc. within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of Alberta and the rules of the U.S. Securities and Exchange Commission.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus or a prospectus supplement relating to the securities purchased by a purchaser and any amendments thereto, irrespective of the determination at a later date of the purchase price of the securities being distributed. In several of the provinces, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revision of the price or damages if the prospectus or a prospectus supplement relating to the securities purchased by a purchaser and any amendments thereto contain a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal adviser. Rights and remedies may also be available to purchasers under U.S. law; purchasers may wish to consult with a U.S. lawyer for particulars of these rights.
Purchasers are cautioned that the statutory right of action for damages for a misrepresentation contained in this prospectus supplement, the base shelf prospectus or any amendment hereto or thereto is limited, in certain provincial securities legislation, to the price at which the Offered Units are offered to the public. This means that, with respect to the Warrants, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon conversion, exchange or exercise of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal advisor.
S-52
In the opinion of Borden Ladner Gervais LLP, Canadian legal counsel to Aurinia Pharmaceuticals, and Stikeman Elliott LLP, Canadian legal counsel to the Underwriters, based on the provisions of the Tax Act, as of the date hereof, the Unit Shares, Warrants and Warrant Shares, if issued on the date hereof, would be “qualified investments” under the Tax Act for trusts governed by a “registered retirement savings plan” (“RRSP”), “registered retirement income fund” (“RRIF”), “deferred profit sharing plan”, “registered education savings plan”, “registered disability savings plan”, or “tax-free savings account” (“TFSA”), as those terms are defined for the purpose of the Tax Act, provided that (i) the Unit Shares and Warrant Shares are listed on a “designated stock exchange” as defined in the Tax Act, which currently includes the TSX and NASDAQ, and (ii) in the case of the Warrants, Aurinia Pharmaceuticals is not a “connected person” under the Plan. For this purpose, a connected person under a Plan is a person who is an annuitant, beneficiary, employer or subscriber under, or a holder of, the Plan and each person who does not deal at arm’s length with that person.
A Unit Share, Warrant or Warrant Share, that is a qualified investment for an RRSP, RRIF or TFSA (a “Registered Plan”) may nevertheless be a “prohibited investment” (within the meaning of the Tax Act) for the Registered Plan, in which case the annuitant or holder of the Registered Plan will be subject to penalty taxes set out in the Tax Act. A Unit Share, Warrant or Warrant Share generally will be a prohibited investment for a Registered Plan if the annuitant or holder of the Registered Plan: (i) does not deal at arm’s length with Aurinia Pharmaceuticals for the purposes of the Tax Act; or (ii) has a “significant interest” (as defined in the Tax Act for purposes of the prohibited investment rules) in Aurinia Pharmaceuticals. Unit Shares and Warrant Shares generally will not be a prohibited investment for a Registered Plan if such securities are “excluded property” (as defined in the Tax Act) for trusts governed by a Registered Plan.
Any person who intends to hold Unit Shares, Warrants or Warrant Shares in a Registered Plan should consult his or her own tax advisers regarding the application of these rules to his or her particular circumstances.
S-53
CERTIFICATE OF AURINIA PHARMACEUTICALS INC.
Dated: December 22, 2016
The short form prospectus, together with the documents incorporated in the prospectus by reference, as supplemented by the foregoing, constitutes full, true and plain disclosure of all material facts relating to the securities offered by the prospectus and this supplement as required by the securities legislation of British Columbia, Alberta and Ontario.
| (signed) Charles A. Rowland, Jr. |
(signed) Dennis Bourgeault | |||
| Charles A. Rowland, Jr. | Dennis Bourgeault | |||
| President and Chief Executive Officer | Chief Financial Officer |
On Behalf of the Board of Directors
| (signed) Richard Glickman |
(signed) Gregory Ayers | |||
| Richard Glickman | Gregory Ayers | |||
| Director | Director |
C-1
CERTIFICATE OF THE UNDERWRITERS
Dated: December 22, 2016
To the best of our knowledge, information and belief, the short form prospectus, together with the documents incorporated in the prospectus by reference, as supplemented by the foregoing, constitutes full, true and plain disclosure of all material facts relating to the securities offered by the prospectus and this supplement as required by the securities legislation of British Columbia, Alberta and Ontario.
CORMARK SECURITIES INC.
| (signed) Marwan Kubursi |
||
| Marwan Kubursi |
C-2
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully for sale and therein only by persons permitted to sell such securities.
A registration statement relating to these securities has been filed with the United States Securities and Exchange Commission. These securities may not be offered nor any offers to buy be accepted prior to the time the registration statement becomes effective. This short form prospectus shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such state.
Information has been incorporated by reference in this short form prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of Aurinia Pharmaceuticals Inc. at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, Canada, Telephone: (604) 632-3473 and are also available electronically at www.sedar.com and www.sec.gov.
SHORT FORM BASE SHELF PROSPECTUS
| New Issue and Secondary Offering | October 16, 2015 |

AURINIA PHARMACEUTICALS INC.
US $250,000,000
Common Shares
Warrants
Subscription Receipts
This short form base shelf prospectus (the “Prospectus”) relates to the issue and sale from time to time, during the 25-month period that this Prospectus, including any amendments hereto, remains effective, of common shares (“Common Shares”) in the capital of Aurinia Pharmaceuticals Inc. (“Aurinia” or the “Company”), warrants to purchase Common Shares (“Warrants”) or subscription receipts that entitle the holder to receive upon satisfaction of certain release conditions, and for no additional consideration, Common Shares (“Subscription Receipts”), (collectively, the “Securities”) or any combination of such Securities in one or more series or issuances, with a total offering price of such Securities, in the aggregate, of up to US$250,000,000 (or its equivalent in Canadian dollars or any other currency). The securities may be offered by the Company or by the Company’s securityholders. The securities may be offered separately or together, in amounts, at prices and on terms to be determined based on market conditions at the time of the sale and set forth in an accompanying prospectus supplement.
The Common Shares are listed on the Nasdaq Stock Market, or NASDAQ, under the symbol “AUPH” and on the Toronto Stock Exchange, or TSX, under the symbol “AUP”. On October 15, 2015, the last trading day prior to the filing of this Prospectus, the closing price of the Common Shares was US$2.93 on NASDAQ and CDN$3.74 on the TSX.
All information permitted under securities legislation to be omitted from this Prospectus will be contained in one or more prospectus supplements (each, a “Prospectus Supplement”) that will be delivered to purchasers together with this Prospectus. Each Prospectus Supplement will be incorporated by reference into this Prospectus for the purposes of
securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains. You should read this Prospectus and any applicable Prospectus Supplement carefully before you invest in any Securities issued pursuant to this Prospectus.
Unless otherwise specified in an applicable Prospectus Supplement, the Company’s Warrants and Subscription Receipts will not be listed on any securities or stock exchanges or on any automated dealer quotation system. There is currently no market through which the Company’s Securities, other than the Common Shares, may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of the Company’s Securities, other than the Common Shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these Securities and the extent of issuer regulation. See “Risk Factors”.
The Securities may be sold pursuant to this Prospectus through underwriters, dealers or agents designated from time to time or directly by the Company at amounts and prices and other terms determined by the Company. In connection with any underwritten offering of the Securities, the underwriters may over-allot or effect transactions which stabilize or maintain the market price of the Securities offered. Such transactions, if commenced, may discontinue at any time. See “Plan of Distribution”. A Prospectus Supplement relating to a particular offering of Securities will set out the names of any underwriters, dealers or agents involved in the sale of the Securities, the amounts, if any, to be purchased by underwriters, the plan of distribution for such Securities, including the net proceeds the Company expects to receive from the sale of such Securities, if any, the amounts and prices at which such Securities are to be sold and the compensation of such underwriters, dealers of agents.
The Company’s registered office is located at #201, 17904 – 105 Avenue, Edmonton, Alberta T5S 2H5, Canada. The Company’s head office is located at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8, Canada.
Agent for Service of Process
Gregory Ayers, Hyuek Joon Lee, David Jayne, Charles Rowland and Stephen Zaruby are directors of the Company and reside outside of Canada. Each of these directors has appointed the following agent for service of process in Canada:
| Name of Person |
Name and Address of Agent | |
| Gregory Ayers, Hyuek Joon Lee, David Jayne, Charles Rowland and Stephen Zaruby |
Borden Ladner Gervais LLP 1200 Waterfront Centre 200 Burrard Street, P.O. Box 48600 Vancouver, BC V7X 1T2 Attention : Stephen P. Robertson |
Investing in the Securities involves a high degree of risk. You should carefully read the “Risk Factors” section beginning on page 8 of this Prospectus.
The Company is permitted under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United States to prepare this Prospectus in accordance with the disclosure requirements of Canada. Prospective investors in the United States should be aware that such requirements are different from those of the United States.
Owning the Securities may subject you to tax consequences both in Canada and the United States. Such tax consequences are not described in this Prospectus and may not be fully described in any applicable Prospectus Supplement. You should read the tax discussion in any Prospectus Supplement with respect to a particular offering and consult your own tax advisor with respect to your own particular circumstances.
Your ability to enforce civil liabilities under the U.S. federal securities laws may be affected adversely because the Company is incorporated under the provincial laws of Alberta, most of the Company’s officers and directors and the experts named in this Prospectus are Canadian residents or residents outside of the United States, and a substantial portion of the Company’s assets and the assets of its officers, directors and experts are located outside of the United States.
Neither the U.S. Securities and Exchange Commission, or SEC, nor any state securities regulator has approved or disapproved the Securities offered hereby or passed upon the accuracy or adequacy of this Prospectus. Any representation to the contrary is a criminal offence.
No underwriter has been involved in the preparation of this Prospectus or performed any review of the contents of this Prospectus.
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 8 | ||||
| 20 | ||||
| 21 | ||||
| 21 | ||||
| 21 | ||||
| 22 | ||||
| 23 | ||||
| 24 | ||||
| 27 | ||||
| 27 | ||||
| 30 | ||||
| 30 | ||||
| 30 | ||||
| 31 | ||||
| 32 | ||||
| 32 | ||||
| 32 | ||||
| 33 | ||||
| 34 | ||||
You should rely only on the information contained or incorporated by reference in this Prospectus and any applicable Prospectus Supplement and on the other information included in the registration statement of which this Prospectus forms a part. The Company has not authorized anyone to provide you with different or additional information. If anyone provides you with different or additional information, you should not rely on it. The Company is not making an offer to sell or seeking an offer to buy the Securities offered pursuant to this Prospectus in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this Prospectus or any applicable Prospectus Supplement is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this Prospectus or any applicable Prospectus Supplement or of any sale of Securities pursuant thereto. The Company’s business, financial condition, results of operations and prospects may have changed since those dates.
Market data and certain industry forecasts used in this Prospectus or any applicable Prospectus Supplement and the documents incorporated by reference in this Prospectus or any applicable Prospectus Supplement were obtained from market research, publicly available information and industry publications. The Company believes that these sources are generally reliable, but the accuracy and completeness of this information is not guaranteed. The Company has not independently verified such information, and the Company does not make any representation as to the accuracy of such information.
In this Prospectus and any Prospectus Supplement, unless otherwise indicated, all dollar amounts and references to “US$” are to U.S. dollars and references to “CDN$” are to Canadian dollars. This Prospectus and the documents incorporated by reference contain translations of some Canadian dollar amounts into U.S. dollars solely for your convenience. See “Exchange Rate Information”.
In this Prospectus and in any Prospectus Supplement, unless the context otherwise requires, references to “Aurinia” or the “Company”, refer to Aurinia Pharmaceuticals Inc., either alone or together with its subsidiaries.
PRESENTATION OF FINANCIAL INFORMATION
The Company’s consolidated financial statements have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board or IASB.
A statement is forward-looking when it uses what the Company knows and expects today to make a statement about the future. Forward-looking statements may include words such as “anticipate”, “believe”, “intend”, “expect”, “goal”, “may”, “outlook”, “plan”, “seek”, “should”, “strive”, “target”, “could”, “continue”, “potential” and “estimated”, or the negative of such terms or comparable terminology. You should not place undue reliance on the forward-looking statements, particularly those concerning anticipated events relating to the development, clinical trials, regulatory approval, and marketing of the Company’s product and the timing or magnitude of those events, as they are inherently risky and uncertain.
Securities laws encourage companies to disclose forward-looking information so that investors can get a better understanding of the Company’s future prospects and make informed investment decisions. These statements, made either in this Prospectus or a document incorporated by reference in this Prospectus, may include, without limitation:
| • | plans to fund the Company’s operations; |
| • | the Company’s intended use of the proceeds from the sale of the Securities; |
| • | statements concerning strategic alternatives and future operations; |
| • | partnering activities; |
| • | summary statements relating to results of the past voclosporin trials or plans to advance the development of voclosporin; |
| • | statements concerning partnership activities and health regulatory discussions; |
| • | the timing of completion of patient enrolment in the Company’s AURA-LV and AURION studies; |
| • | the timing of commencement and completion of clinical trials; |
| • | the Company’s intention to seek regulatory approvals in the United States and Europe for voclosporin; |
| • | the Company’s intention to seek additional corporate alliances and collaborative agreements to support the commercialization and development of its product; |
| • | the Company’s intention to demonstrate that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class status for the treatment of LN (“lupus nephritis”) outside of Japan; |
| • | the Company’s intention to use the LN Phase 2b clinical trial program to gain a clearer understanding of voclosporin’s time to onset of action in patients suffering from LN; |
| • | the Company’s belief that recent granted formulation patents regarding the delivery of voclosporin to the ocular surface for conditions such as dry eye have the potential to be of therapeutic value; |
| • | the Company’s belief that voclosporin has further potential to be of therapeutic value in other autoimmune indications and in the prevention of transplant rejection; |
| • | the Company’s intention to seek regulatory approval in other jurisdictions in the future and initiate clinical studies; |
| • | the Company’s anticipated future financial position, future revenues and projected costs; and |
| • | plans and objectives of management. |
Such statements reflect the Company’s current views with respect to future events and are subject to risks and uncertainties and are necessarily based on a number of estimates and assumptions that, while considered reasonable by the Company, as at the date of such statements, are inherently subject to significant business, economic, competitive, political, scientific and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The factors and assumptions used by the Company to develop such forward-looking statements include, but are not limited to: the assumption that the Company will be able to reach agreements with regulatory agencies on executable development programs; the assumption that recruitment to clinical trials will occur as projected; the assumption that the Company will successfully complete its clinical programs on a timely basis, including the Phase 2b LN clinical trial currently in progress, to enable the Company to proceed to conduct the required Phase 3 LN clinical trials and meet regulatory requirements for approval of marketing authorization applications and new drug approvals; the assumption the regulatory requirements will be maintained; the assumption that the Company will be able to manufacture and secure a sufficient supply of voclosporin to successfully complete the development and commercialization of voclosporin; the assumption that the Company’s patent portfolio is sufficient and valid; the assumption that there is a potential commercial value for other indications for voclosporin; the assumption that market data and reports reviewed by the Company are accurate; the assumption that the Company’s current good relationships with its suppliers, service providers and other third parties will be maintained; the assumptions relating to the availability of capital on terms that are favourable to the Company; the assumption that the Company will be able to attract and retain skilled staff; the assumption that general business and economic conditions will be maintained, and the assumptions relating to the feasibility of future clinical trials.
It is important to know that:
| • | Actual results could be materially different from what the Company expects if known or unknown risks affect its business, or if the Company’s estimates or assumptions turn out to be inaccurate. As a result, |
2
| the Company cannot guarantee that any forward-looking statement will materialize and, accordingly, you are cautioned not to place undue reliance on these forward-looking statements. |
| • | Forward-looking statements do not take into account the effect that transactions or non-recurring or other special items announced or occurring after the statements are made may have on the Company’s business. For example, they do not include the effect of mergers, acquisitions, other business combinations or transactions, dispositions, sales of assets, asset write-downs or other charges announced or occurring after the forward-looking statements are made. The financial impact of such transactions and non-recurring and other special items can be complex and necessarily depends on the facts particular to each of them. Accordingly, the expected impact cannot be meaningfully described in the abstract or presented in the same manner as known risks affecting the Company’s business. |
| • | The Company disclaims any intention and assumes no obligation to update any forward-looking statements even if new information becomes available, as a result of future events, new information, or for any other reason except as required by law. |
The factors discussed below and other considerations discussed in the “Risk Factors” section of this Prospectus could cause the Company’s actual results to differ significantly from those contained in any forward-looking statements.
Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause the Company’s actual results, performance, or achievements to differ materially from any further results, performance or achievements expressed or implied by such forward-looking statements. Important factors that could cause such differences include, among other things, the following:
| • | the need for additional capital in the longer term to fund the Company’s development programs and the effect of capital market conditions and other factors on capital availability; |
| • | difficulties, delays, or failures the Company may experience in the conduct of and reporting of results of its clinical trials for voclosporin, and in particular its current LN Phase 2b clinical trial; |
| • | difficulties, delays or failures in obtaining regulatory approvals for the initiation of clinical trials; |
| • | difficulties, delays or failures in obtaining regulatory approvals to market voclosporin; |
| • | difficulties the Company may experience in completing the development and commercialization of voclosporin; |
| • | insufficient acceptance of and demand for voclosporin; |
| • | difficulties, delays, or failures in obtaining appropriate reimbursement of voclosporin; and/or |
| • | difficulties that the Company may experience in identifying and successfully securing appropriate corporate alliances to support the development and commercialization of its product. |
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, levels of activity, performance or achievements. These forward-looking statements are made as of the date of this Prospectus or, in the case of documents incorporated by reference in this Prospectus, as of the date of such documents or, in the case of any Prospectus Supplement, as of the date of such Prospectus Supplement and the Company disclaims any intention and have no obligation or responsibility, except as required by law, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
3
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada which have also been filed with, or furnished to, the United States Securities and Exchange Commission (the “SEC”). Copies of the documents incorporated by reference in this Prospectus and not delivered with this Prospectus may be obtained on request without charge from the Company’s Corporate Secretary at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, Canada, Telephone: (604) 632-3473 or by accessing the disclosure documents through the Internet on the Canadian System for Electronic Analysis and Retrieval, or SEDAR, at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC’s Electronic Data Gathering and Retrieval System, or EDGAR, at www.sec.gov.
The following documents, filed with the securities commissions or similar regulatory authorities in British Columbia, Alberta and Ontario, and filed with, or furnished to, the SEC are specifically incorporated by reference into, and form an integral part of, this Prospectus:
| (a) | the annual information form of the Company dated March 26, 2015 for the fiscal year ended December 31, 2014 (the “AIF”); |
| (b) | the amended audited consolidated balance sheets of the Company as at December 31, 2014 and 2013, and the consolidated statements of operations, changes in shareholders’ equity and cash flows for each of the years in the two-year period ended December 31, 2014, including the notes thereto and the auditors’ report thereon, as filed on May 15, 2015; |
| (c) | the amended Management’s Discussion and Analysis of Financial Condition and Results of Operations for the year ended December 31, 2014, as filed on May 15, 2015; |
| (d) | the unaudited comparative consolidated interim financial statements of the Company as at and for the period ended March 31, 2015; |
| (e) | Management’s Discussion and Analysis of Financial Condition and Results of Operations for the period ended March 31, 2015; |
| (f) | the unaudited comparative consolidated interim financial statements of the Company as at and for the period ended June 30, 2015; |
| (g) | Management’s Discussion and Analysis of Financial Condition and Results of Operations for the period ended June 30, 2015; and |
| (h) | the management information circular dated April 24, 2015 in connection with the annual general meeting of Aurinia’s shareholders held on May 26, 2015. |
Any documents of the type described in Section 11.1 of Form 44-101F1 Short Form Prospectuses filed by the Company with a securities commission or similar authority in any jurisdiction of Canada subsequent to the date of this Prospectus and prior to the expiry of this Prospectus, or the completion of the issuance of Securities pursuant hereto, will be deemed to be incorporated by reference into this Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus is filed with, or furnished to, the SEC pursuant to the Securities Exchange Act of 1934, as amended (the “Exchange Act”), after the date of this Prospectus, such document or information will be deemed to be incorporated by reference as an exhibit to the registration statement of which this Prospectus forms a part (in the case of a report on Form 6-K, if and to the extent expressly provided therein).
A Prospectus Supplement containing the specific terms of any offering of the Securities will be delivered to purchasers of such Securities together with this Prospectus and will be deemed to be incorporated by reference in this Prospectus as of the date of such Prospectus Supplement and only for the purposes of the offering of the Securities to which that Prospectus Supplement pertains.
4
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded, for the purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this Prospectus.
Upon filing a new annual information form and the related annual financial statements and management’s discussion and analysis with applicable securities regulatory authorities during the currency of this Prospectus, the previous annual information form, the previous annual financial statements and management’s discussion and analysis and all quarterly financial statements, supplemental information, material change reports and information circulars filed prior to the commencement of the Company’s financial year in which the new annual information form is filed will be deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of the Securities under this Prospectus. Upon interim consolidated financial statements and the accompanying management’s discussion and analysis being filed by the Company with the applicable securities regulatory authorities during the duration of this Prospectus, all interim consolidated financial statements and the accompanying management’s discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of Securities under this Prospectus.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been or will be filed with the SEC as part of the registration statement of which this Prospectus forms a part: (i) the documents listed under the heading “Documents Incorporated by Reference”; (ii) powers of attorney from the Company’s directors and officers; and (iii) the consent of PricewaterhouseCoopers LLP.
5
The following table sets forth for each period indicated: (i) the noon exchange rates in effect at the end of the period; (ii) the high and low noon exchange rates during such period; and (iii) the average noon exchange rates for such period, for one Canadian dollar, expressed in U.S. dollars, as quoted by the Bank of Canada.
| Year Ended December 31 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| US$ | US$ | US$ | ||||||||||
| Closing |
0.8620 | 0.9402 | 1.0051 | |||||||||
| High |
0.9422 | 1.0164 | 1.0299 | |||||||||
| Low |
0.8589 | 0.9348 | 0.9599 | |||||||||
| Average |
0.9054 | 0.9710 | 1.0004 | |||||||||
| Six Months Ended June 30 | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| US$ | US$ | US$ | ||||||||||
| Closing |
0.8006 | 0.9372 | 0.9508 | |||||||||
| High |
0.8527 | 0.9422 | 1.0164 | |||||||||
| Low |
0.7811 | 0.8888 | 0.9495 | |||||||||
| Average |
0.8095 | 0.9117 | 0.9844 | |||||||||
On October 15, 2015, the noon exchange rate as quoted by the Bank of Canada was CDN$1.00 = US$0.7750.
The Company is a clinical stage pharmaceutical company with its registered office located at #201, 17904 – 105 Avenue, Edmonton, Alberta T5S 2H5, Canada. The Company’s head office is located at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8, Canada and incorporates the clinical, regulatory and business development functions of the Company. The office of the Chief Executive Officer is located in Bellevue, Washington, U.S.A.
Aurinia Pharmaceuticals Inc. is organized under the Business Corporations Act (Alberta).
The Company’s Common Shares are currently listed and traded on the NASDAQ under the symbol “AUPH” and on the TSX under the symbol “AUP”.
The Company has the following wholly-owned subsidiaries: Aurinia Pharma Corp. (British Columbia incorporated), Aurinia Pharmaceuticals, Inc. (Delaware incorporated) and Aurinia Pharma Limited (UK incorporated).
The Company’s By-Law No. 2 was amended at a shareholder’s meeting held on August 15, 2013 to include provisions requiring advance notice for any nominations of directors by shareholders.
SUMMARY DESCRIPTION OF BUSINESS
Aurinia is focused on the development of its novel therapeutic immunomodulating drug candidate, voclosporin, which is a next generation calcineurin inhibitor (“CNI”). It has been studied in kidney rejection following transplantation, psoriasis and in various forms of uveitis (an ophthalmic disease).
6
The Company has, since September 20, 2013, rebranded, restructured and refocused itself around a strategy that focuses on the development of voclosporin for the treatment of LN. The mechanism of action of voclosporin, a CNI, has been validated with certain first generation CNIs for the prevention of rejection in patients undergoing solid organ transplants and in several autoimmune indications, including dermatitis, keratoconjunctivitis sicca (Dry Eye Syndrome), psoriasis, rheumatoid arthritis, and for LN in Japan. The Company believes that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class regulatory approval status for the treatment of LN outside of Japan.
The Company will also continue to evaluate opportunities for other indications of voclosporin to create shareholder value.
In February 2014, the Company completed a private placement with net proceeds of $48.31 million, the net proceeds of which were to be used to advance the clinical and non-clinical development of its lead drug voclosporin, as a therapy for LN, and for general corporate purposes. A summary of the anticipated and actual use of proceeds up to and including June 30, 2015 from that financing are set out below (other than working capital):
| Expected use of proceeds for period to June 30, 2015 (in thousands) $ |
Incurred for period to June 30, 2015 (in thousands) $ |
|||||||
| Research and development of voclosporin |
18,509 | 16,319 | ||||||
|
|
|
|
|
|||||
| Other corporate purposes |
||||||||
| Corporate, administration, business development |
7,760 | 6,851 | ||||||
| Repayment of drug supply loan |
1,290 | 1,290 | ||||||
| Payment of financing milestone to ILJIN |
1,472 | 1,600 | ||||||
|
|
|
|
|
|||||
| 10,522 | 9,741 | |||||||
|
|
|
|
|
|||||
For the period from the date of the private placement to June 30, 2015, the actual use of proceeds was slightly less than the original estimates. This is primarily the result of actual voclosporin Phase 2b clinical trial expenditures to date being less than originally estimated due to the difference in timing of these expenditures. There is not expected to be any significant impact on the Company’s ability to achieve its business objectives and milestones as a result of this variation.
7
Investing in Securities involves a high degree of risk. In addition to the other information included or incorporated by reference in this Prospectus or any applicable Prospectus Supplement, you should carefully consider the risks described below before purchasing Securities. If any of the following risks actually occur, the Company’s business, financial condition and results of operations could materially suffer. As a result, the trading price of Common Shares could decline, and you might lose all or part of your investment. The risks set out below are not the only risks the Company faces; risks and uncertainties not currently known to the Company or that the Company currently deems to be immaterial may also materially and adversely affect the Company’s business, financial condition and results of operations. You should also refer to the other information set forth or incorporated by reference in this Prospectus or any applicable Prospectus Supplement, including the Company’s consolidated financial statements and related notes.
Risks Relating to Aurinia’s Business
Clinical Trial Progress and Results – Heavy Dependence on Voclosporin
The Company has invested a significant portion of its time and financial resources in the development of voclosporin. Voclosporin is currently the Company’s only product. The Company anticipates that its ability to generate revenues and meet expectations will depend on the successful development and commercialization of voclosporin. The successful development and commercialization of voclosporin will depend on several factors, including the following:
| • | successful completion of clinical programs, and in particular, the Phase 2b LN clinical trial currently in progress; |
| • | receipt of marketing approvals from the FDA and other regulatory authorities with a commercially viable label; |
| • | securing and maintaining partners with sufficient expertise and resources to help in the continuing development and eventual commercialization of voclosporin for autoimmune indications and/or transplant; |
| • | maintaining suitable manufacturing and supply agreements to ensure commercial quantities of the product through validated processes; and |
| • | acceptance and adoption of the product by the medical community and third-party payors. |
It is possible that the Company may decide to discontinue the development of voclosporin at any time for commercial, scientific, or regulatory reasons. If voclosporin is developed, but not marketed, the Company will have invested significant resources and its future operating results and financial conditions would be significantly adversely affected. If the Company is not successful in commercializing voclosporin, or significantly delayed in doing so, its business will be materially harmed and the Company may need to curtail or cease operations.
Product Development Goals and Time Frames
The Company sets goals for, and makes public statements regarding, timing of the accomplishment of objectives material to its success, such as the commencement and completion of clinical trials, anticipated regulatory approval dates, and time of product launch. The actual timing of these events can vary dramatically due to factors such as delays or failures in clinical trials, the uncertainties inherent in the regulatory approval process, and delays in achieving product development, manufacturing, or marketing milestones necessary to commercialize its product. There can be no assurance that the Company’s clinical trials will be completed, that regulatory submissions will be made or receive regulatory approvals as planned, or that the Company will be able to adhere to the current schedule for the validation of manufacturing and launch of its product. If the Company fails to achieve one or more of these milestones as planned, the price of the Company’s Securities could decline.
8
No Assurance of Successful Development
The Company has not completed the development of any therapeutic products and in particular, voclosporin, and therefore there can be no assurance that any product will be successfully developed. The Company’s therapeutic product has not received regulatory approval for commercial use and sale for any indication, in any jurisdiction. The Company cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. In general, significant research and development and clinical studies are required to demonstrate the safety and effectiveness of its products before submission of any regulatory applications. The Company may never obtain the required regulatory approvals for its product in any indication. Product candidates require significant additional research and development efforts, including clinical trials, prior to regulatory approval and potential commercialization, however, there can be no assurance that the results of all required clinical trials will demonstrate that these product candidates are safe and effective or, even if the results of all required clinical trials do demonstrate that these product candidates are safe and effective, or even if the results of the clinical trials are considered successful by the Company, that the regulatory authorities will not require the Company to conduct additional clinical trials before they will consider approving such product candidates for commercial use. Approval or consent by regulatory authorities to commence a clinical trial does not indicate that the device, drug, or treatment being studied can or will be approved. Preparing, submitting, and advancing applications for regulatory approval is complex, expensive, time intensive and entails significant uncertainty.
The results of the Company’s completed preclinical studies and clinical trials may not be indicative of future clinical trial results. A commitment of substantial resources to conduct time-consuming research, preclinical studies, and clinical trials will be required if the Company is to complete the development of its product.
There can be no assurance that unacceptable toxicities or adverse side effects will not occur at any time in the course of preclinical studies or human clinical trials or, if any products are successfully developed and approved for marketing, during commercial use of its products. The appearance of any such unacceptable toxicities or adverse side effects could interrupt, limit, delay, or abort the development of the Company’s product or, if previously approved, necessitate their withdrawal from the market. Furthermore, there can be no assurance that disease resistance or other unforeseen factors will not limit the effectiveness of the Company’s product. Any products resulting from the Company’s programs are not expected to be successfully developed or made commercially available in the near term and may not be successfully developed or made commercially available at all. Should the Company’s product prove to have insufficient benefit and/or have an unsafe profile, its development will likely be discontinued.
The future performance of the Company will be impacted by a number of important factors, including, in the short-term, its ability to continue to generate cash flow from financings, and in the longer term, its ability to generate royalty or other revenues from licensed technology and bring new products to the market. The Company’s future success will require efficacy and safety of its product and regulatory approval for the product. Future success of commercialization of any product is also dependant on the ability of the Company to obtain patents, enforce such patents, avoid patent infringement, and obtain patent extensions where applicable.
9
The Company will have significant additional future capital needs and there are uncertainties as to the Company’s ability to raise additional funding.
The Company will require significant additional capital resources to expand the Company’s business, in particular the further development of the Company’s product candidate, voclosporin. Advancing the Company’s product candidate, market for the Company’s product, or acquisition and development of any new products or product candidates will require considerable resources and additional access to capital markets. In addition, the Company’s future cash requirements may vary materially from those now expected. For example, the Company’s future capital requirements may increase if:
| • | the Company experiences unexpected or increased costs relating to preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, or other lawsuits, brought by either the Company or its competition; |
| • | the Company experiences scientific progress sooner than expected in its discovery, research and development projects, if the Company expands the magnitude and scope of these activities, or if the Company modifies the Company’s focus as a result of its discoveries; |
| • | the Company is required to perform additional pre-clinical studies and clinical trials; or |
| • | the Company elects to develop, acquire or license new technologies, products or businesses. |
The Company could potentially seek additional funding through corporate collaborations and licensing arrangements or through public or private equity or debt financing. However, if capital market conditions in general, or with respect to life sciences companies such as the Company, are unfavourable, the Company’s ability to obtain significant additional funding on acceptable terms, if at all, will be negatively affected. Additional financing that the Company may pursue may involve the sale of Common Shares which could result in significant dilution to the Company’s shareholders.
If sufficient capital is not available, the Company may be required to delay the Company’s research and development projects, which could have a material adverse effect on the Company’s business, financial condition, prospects or results of operations.
Negative Cash Flow
The Company had negative operating cash flow for the financial year ended December 31, 2014. The Company anticipates that it will continue to have negative cash flow as it continues its development of voclosporin. To the extent that the Company has negative operating cash flow in future periods, it may need to allocate a portion of its cash reserves to fund such negative cash flow. The Company may also be required to raise additional funds through the issuance of equity or debt securities. There can be no assurance that the Company will be able to generate a positive cash flow from its operations, that additional capital or other types of financing will be available when needed or that these financings will be on terms favourable to the Company.
Patents and Proprietary Technology
Patents and other proprietary rights are essential to the Company’s business. The Company’s policy has been to file patent applications to protect technology, inventions, and improvements to its inventions that are considered important to the development of its business.
The Company’s success will depend in part on its ability to obtain patents, defend patents, maintain trade secret protection and operate without infringing on the proprietary rights of others. Interpretation and evaluation of pharmaceutical patent claims present complex and often novel legal and factual questions. Accordingly, there is some question as to the extent to which biopharmaceutical discoveries and related products and processes can be effectively protected by patents. As a result, there can be no assurance that:
| • | patent applications will result in the issuance of patents; |
10
| • | additional proprietary products developed will be patentable; |
| • | patents issued will provide adequate protection or any competitive advantages; |
| • | patents issued will not be successfully challenged by third parties; |
| • | the patents issued do not infringe the patents or intellectual property of others; or |
| • | that the Company will be able to obtain any extensions of the patent term. |
A number of pharmaceutical, biotechnology, medical device companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to the business of the Company. Some of these technologies, applications or patents may conflict with or adversely affect the technologies or intellectual property rights of the Company. Any conflicts with the intellectual property of others could limit the scope of the patents, if any, that the Company may be able to obtain or result in the denial of patent applications altogether.
Further, there may be uncertainty as to whether the Company may be able to successfully defend any challenge to its patent portfolio. Moreover, the Company may have to participate in interference proceedings in the various jurisdictions around the world. An unfavorable outcome in an interference or opposition proceeding could preclude the Company or its collaborators or licensees from making, using or selling products using the technology, or require the Company to obtain license rights from third parties. It is not known whether any prevailing party would offer a license on commercially acceptable terms, if at all. Further, any such license could require the expenditure of substantial time and resources and could harm the business of the Company. If such licenses are not available, the Company could encounter delays or prohibition of the development or introduction of the product of the Company.
Clinical trials for the Company’s product candidates are expensive and time-consuming, and their outcome is uncertain.
Before the Company can obtain regulatory approval for the commercial sale of any product candidate currently under development, the Company is required to complete extensive clinical trials to demonstrate its safety and efficacy. Clinical trials are very expensive and difficult to design and implement. The clinical trial process is also time-consuming. If the Company finds a collaboration partner for the development of voclosporin, the clinical trials are expected to continue for several years, although costs associated with voclosporin may well be shared with the Company’s collaboration partner. The timing of the commencement, continuation and completion of clinical trials may be subject to significant delays relating to various causes, including:
| • | the Company’s inability to find collaboration partners; |
| • | the Company’s inability to manufacture or obtain sufficient quantities of materials for use in clinical trials; |
| • | delays in obtaining regulatory approvals to commence a study, or government intervention to suspend or terminate a study; |
| • | delays, suspension, or termination of the clinical trials imposed by the institutional review board or independent ethics board responsible for overseeing the study to protect research subjects at a particular study site; |
| • | delays in identifying and reaching agreement on acceptable terms with prospective clinical trial sites; |
| • | slower than expected rates of patient recruitment and enrollment; |
| • | uncertain dosing issues; |
| • | inability or unwillingness of medical investigators to follow the Company’s clinical protocols; |
| • | variability in the number and types of subjects available for each study and resulting difficulties in identifying and enrolling subjects who meet trial eligibility criteria; |
11
| • | scheduling conflicts with participating clinicians and clinical institutions; |
| • | difficulty in maintaining contact with subjects after treatment, which results in incomplete data; |
| • | unforeseen safety issues or side effects; |
| • | lack of efficacy during the clinical trials; |
| • | the Company’s reliance on clinical research organizations to conduct clinical trials, which may not conduct those trials with good clinical or laboratory practices; or |
| • | other regulatory delays. |
The results of pre-clinical studies and initial clinical trials are not necessarily predictive of future results, and the Company’s current product candidate may not have favourable results in later trials or in the commercial setting.
Pre-clinical tests and Phase 1 and Phase 2 clinical trials are primarily designed to test safety, to study pharmacokinetics and pharmacodynamics and to understand the side effects of product candidates at various doses and schedules. Success in pre-clinical or animal studies and early clinical trials does not ensure that later large scale efficacy trials will be successful nor does it predict final results. Favourable results in early trials may not be repeated in later trials.
A number of companies in the life sciences industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events and could cause a clinical trial to be repeated or terminated. Pre-clinical data and the clinical results the Company has obtained for voclosporin may not predict results from studies in larger numbers of subjects drawn from more diverse populations or in a commercial setting, and also may not predict the ability of the Company’s product to achieve its intended goals, or to do so safely.
The Company will be required to demonstrate through larger-scale clinical trials that voclosporin is safe and effective for use in a diverse population before the Company can seek regulatory approvals for its commercial sale. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical and post-approval trials. If voclosporin fails to demonstrate sufficient safety and efficacy in ongoing or future clinical trials, the Company could experience potentially significant delays in, or be required to abandon development of, the Company’s product candidate currently under development.
The Company’s industry is subject to health and safety risks.
The Company produces a product for human ingestion. While the Company takes substantial precautions such as laboratory and clinical testing, toxicology studies, quality control and assurance testing and controlled production methods, the associated health and safety risks cannot be eliminated. Products produced by the Company may be found to be, or to contain substances that are harmful to the health of the Company’s patients and customers and which, in extreme cases, may cause serious health conditions or death. This sort of finding may expose the Company to substantial risk of litigation and liability.
Further, the Company would be forced to discontinue production of the Company’s product, which would harm the Company’s profitability. Aurinia maintains product liability insurance coverage; however, there is no guarantee that the Company’s current coverage will be sufficient or that the Company can secure insurance coverage in the future at commercially viable rates or with the appropriate limits.
12
The Company’s product may not achieve or maintain expected levels of market acceptance, which could have a material adverse effect on the Company’s business, financial condition and results of operations and could cause the market value of the Company’s Securities to decline.
Even if the Company is able to obtain regulatory approvals for the Company’s product, the success of the product is dependent upon achieving and maintaining market acceptance. New product candidates that appear promising in development may fail to reach the market or may have only limited or no commercial success. Levels of market acceptance for the Company’s product could be impacted by several factors, many of which are not within the Company’s control, including but not limited to:
| • | safety, efficacy, convenience and cost-effectiveness of the Company’s product compared to products of the Company’s competitors; |
| • | scope of approved uses and marketing approval; |
| • | timing of market approvals and market entry; |
| • | difficulty in, or excessive costs to, manufacture; |
| • | infringement or alleged infringement of the patents or intellectual property rights of others; |
| • | availability of alternative products from the Company’s competitors; |
| • | acceptance of the price of the Company’s product; and |
| • | ability to market the Company’s product effectively at the retail level. |
In addition, by the time any products are ready to be commercialized, what the Company believes to be the market for these products may have changed. The Company’s estimates of the number of patients who have received or might have been candidates to use a specific product may not accurately reflect the true market or market prices for such products or the extent to which such products, if successfully developed, will actually be used by patients. The Company’s failure to successfully introduce and market its product that are under development would have a material adverse effect on its business, financial condition, and results of operations.
The Company is dependent upon the Company’s key personnel to achieve the Company’s business objectives.
As a technology-driven company, intellectual input from key management and personnel is critical to achieve the Company’s business objectives. Consequently, the Company’s ability to retain these individuals and attract other qualified individuals is critical to the Company’s success. The loss of the services of key individuals might significantly delay or prevent achievement of the Company’s business objectives. In addition, because of a relative scarcity of individuals with the high degree of education and scientific achievement required for the Company’s business, competition among life sciences companies for qualified employees is intense and, as a result, the Company may not be able to attract and retain such individuals on acceptable terms, or at all. In addition, because the Company does not maintain “key person” life insurance on any of the Company’s officers, employees, or consultants, any delay in replacing such persons, or an inability to replace them with persons of similar expertise, would have a material adverse effect on the Company’s business, financial condition, and results of operations.
The Company also has relationships with scientific collaborators at academic and other institutions, some of whom conduct research at the Company’s request or assist the Company in formulating its research and development strategies. These scientific collaborators are not the Company’s employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to the Company. In addition, even though the Company’s collaborators are required to sign confidentiality agreements prior to working with the Company, they may have arrangements with other companies to assist such other companies in developing technologies that may prove competitive to the Company.
13
Incentive provisions for the Company’s key executives include the granting of stock options that vest over time, designed to encourage such individuals to stay with the Company. However, a low share price, whether as a result of disappointing progress in the Company’s development programs or as a result of market conditions generally, could render such agreements of little value to the Company’s key executives. In such event, the Company’s key executives could be susceptible to being hired away by the Company’s competitors who could offer a better compensation package. If the Company is unable to attract and retain key personnel the Company’s business, financial conditions and results of operations may be adversely affected.
The Company is exposed to risks relating to the write-down of intangible assets, which comprises a significant portion of the Company’s total assets.
A significant amount of the Company’s total assets relate to the Company’s intellectual property. As of June 30, 2015, the carrying value of the Company’s intangible assets was approximately US$17.8 million. In accordance with IFRS, the Company is required to review the carrying value of the Company’s intangible assets for impairment periodically or when certain triggers occur. Such impairment will result in a write-down of the intangible asset and the write-down is charged to income during the period in which the impairment occurs. The write-down of any intangible assets could have a material adverse effect on the Company’s business, financial condition, and results of operations.
If the Company were to lose the Company’s foreign private issuer status under U.S. federal securities laws, the Company would likely incur additional expenses associated with compliance with the U.S. securities laws applicable to U.S. domestic issuers.
As a foreign private issuer, as defined in Rule 3b-4 under the Exchange Act, the Company is exempt from certain of the provisions of the U.S. federal securities laws. For example, the U.S. proxy rules and the Section 16 reporting and “short swing” profit rules do not apply to foreign private issuers. However, if the Company were to lose the Company’s status as a foreign private issuer, these regulations would immediately apply and the Company would also be required to commence reporting on forms required of U.S. companies, such as Forms 10-K, 10-Q and 8-K, rather than the forms currently available to the Company, such as Forms 40-F and 6-K. Compliance with these additional disclosure and timing requirements under these securities laws would likely result in increased expenses and would require the Company’s management to devote substantial time and resources to comply with new regulatory requirements. Further, to the extent that the Company was to offer or sell the Company’s Securities outside of the United States, the Company would have to comply with the more restrictive Regulation S requirements that apply to U.S. companies, and the Company would no longer be able to utilize the multijurisdictional disclosure system forms for registered offerings by Canadian companies in the United States, which could limit the Company’s ability to access the capital markets in the future.
Legislative actions, potential new accounting pronouncements, and higher insurance costs are likely to impact the Company’s future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected revenue fluctuations and affect the Company’s financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with greater frequency and are expected to occur in the future. Compliance with changing regulations of corporate governance and public disclosure may result in additional expenses. All of these uncertainties are leading generally toward increasing insurance costs, which may adversely affect the Company’s business, results of operations and the Company’s ability to purchase any such insurance, at acceptable rates or at all, in the future.
The Company relies on third parties for the supply and manufacture of voclosporin, which can be unpredictable in terms of quality, cost and availability.
The Company’s drug, voclosporin, requires a specialized manufacturing process. Lonza Ltd. is currently the sole source manufacturer of voclosporin.
14
The FDA and other regulatory authorities require that drugs be manufactured in accordance with the current good manufacturing practices regulations, as established from time to time. Accordingly, in the event the Company receives marketing approvals for voclosporin, it may need to rely on a limited number of third parties to manufacture and formulate voclosporin. The Company may not be able to arrange for its product to be manufactured on reasonable terms or in sufficient quantities.
Manufacturers of pharmaceutical products often encounter difficulties in production, especially in scaling up initial production. These problems include difficulties with production costs and yields, stability, quality control and assurance, and shortages of qualified personnel, as well as compliance with strictly enforced federal, provincial and foreign regulations. The Company relies on a limited number of third parties to manufacture and supply raw materials for its product. The third parties the Company chooses to manufacture and supply raw materials for its product are not under its control, and may not perform as agreed or may terminate their agreements with the Company, and the Company may not be able to find other third parties to manufacture and supply raw materials on commercially reasonable terms, or at all. If either of these events were to occur, the Company’s operating results and financial condition would be adversely affected.
In addition, drug and chemical manufacturers are subject to various regulatory inspections, including those conducted by the FDA, to ensure strict compliance with good manufacturing practices (“GMP”) and other government regulations. While the Company is obligated to audit the performance of the Company’s third-party contractors, the Company does not have complete control over their compliance. The Company could be adversely impacted if the Company’s third-party manufacturers do not comply with these standards and regulations. For non-compliance, the regulatory authority may levy penalties and sanctions, including fines, injunctions, civil penalties, failure of the government to grant review of submissions or market approval of drugs, or cause delays, suspension or withdrawal of approvals, product seizures or recalls, operating restrictions, facility closures and criminal prosecutions. Any of this will have a material adverse impact on the Company’s business, financial condition, and results of operations.
Anticipated Revenues may be derived from Licensing Activities
The Company anticipates that its revenues in the future may be derived from products licensed to pharmaceutical and biotechnology companies. Accordingly, these revenues will depend, in large part, upon the success of these companies, and the Company’s operating results may fluctuate substantially due to reductions and delays in their research, development and marketing expenditures. These reductions and delays may result from factors that are not within the Company’s control, including:
| • | changes in economic conditions; |
| • | changes in the regulatory environment, including governmental pricing controls affecting health care and health care providers; |
| • | pricing pressures; and |
| • | other factors affecting research and development spending. |
Lack of Operating Profits
The Company has incurred losses and anticipates that its losses will increase as it continues its development and clinical trials and seeks regulatory approval for the sale of its therapeutic product. There can be no assurance that it will have earnings or positive cash flow in the future.
As at June 30, 2015, the Company had an accumulated deficit of $248.48 million. The net operating losses over the near-term and the next several years are expected to continue as a result of initiating new clinical trials and activities necessary to support regulatory approval and commercialization of its product. There can be no assurance that the Company will be able to generate sufficient product revenue to become profitable at all or on a
15
sustained basis. The Company expects to have quarter-to-quarter fluctuations in expenses, some of which could be significant, due to research, development, and clinical trial activities, as well as regulatory and commercialization activities.
The Company’s business depends heavily on the use of information technologies.
Several key areas of the Company’s business depend on the use of information technologies, including production, manufacturing and logistics, as well as clinical and regulatory matters. Despite the Company’s best efforts to prevent such behavior, third parties may nonetheless attempt to hack into the Company’s systems and obtain data relating to the Company’s pre-clinical studies, clinical trials, patients using the Company’s product or the Company’s proprietary information on voclosporin. If the Company fails to maintain or protect the Company’s information systems and data integrity effectively, the Company could lose existing customers, have difficulty attracting new customers, have problems in determining product cost estimates and establishing appropriate pricing, have difficulty preventing, detecting, and controlling fraud, have disputes with customers, physicians, and other health care professionals, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenues as a result of a data privacy breach, or suffer other adverse consequences. While the Company has invested in the protection of data and information technology, there can be no assurance that the Company’s efforts or those of the Company’s third-party collaborators, if any, or manufacturers, to implement adequate security and quality measures for data processing would be sufficient to protect against data deterioration or loss in the event of a system malfunction, or to prevent data from being stolen or corrupted in the event of a security breach. Any such loss or breach could have a material adverse effect on the Company’s business, operating results and financial condition.
Competition and Technological Change
The industry in which the Company operates is highly competitive and the Company has numerous domestic and foreign competitors, including major pharmaceutical and chemical companies, specialized biotechnology companies, universities, academic institutions, government agencies, public and private research organizations and large, fully-integrated pharmaceutical companies which have extensive resources and experience in research and development, process development, clinical evaluation, manufacturing, regulatory affairs, distribution and marketing. Many of the Company’s potential competitors possess substantially greater research and development skills, financial, technical and marketing expertise and human resources than the Company, and may be better equipped to develop, manufacture and market products. There is a risk that new products and technologies may be developed which may be more effective or commercially viable than the product being developed or marketed by the Company, thus making the Company’s product non-competitive or obsolete. There may also be market resistance to the acceptance of the Company’s new product in any indication and a risk that the product, even though clinically effective, is not economically viable in the commercial production stage.
Reliance on Partners
The Company’s strategy and success for the research, development, and commercialization of voclosporin in China, Canada, South Africa and Israel is dependent upon the Company’s partners performing their respective contractual responsibilities. The Company has partnered with 3SBio, Inc. in China and Paladin Labs Inc. in Canada, South Africa and Israel. The amount and timing of resources such partners will devote to these activities may not be within the Company’s control. There can be no assurance that its partners will perform their obligations as expected.
The license, research and development agreements with the partners noted above include indemnification and obligation provisions that are customary in the industry. These guarantees generally require the Company to compensate the other party for certain damages and costs incurred as a result of third party claims or damages arising from these transactions. These provisions may survive termination of the underlying agreement. The nature of the potential obligations prevents the Company from making a reasonable estimate of the maximum potential amount it could be required to pay.
16
Reliance on Other Third Parties
The Company depends on third parties for the sourcing of components or for the product itself. Furthermore, as with other pharmaceutical companies, the Company relies on medical institutions for testing and clinically validating its prospective product. The Company does not anticipate any difficulties in obtaining required components or products or any difficulties in the validation and clinical testing of its product but there is no guarantee that they will be obtained.
The Company currently relies on contract research organizations (“CROs”) for the conduct of its clinical trials. These CROs operate in accordance with good clinical management practices mandated by the regulatory authorities and are subject to regular audits by regulatory authorities and by the Company.
The Company also has arrangements for the encapsulation, packaging and labeling of voclosporin through a third party supplier. Contract manufacturers must operate in compliance with regulatory requirements. Failure to do so could result in, among other things, the disruption of product supplies.
Marketing and Distribution
The Company has limited experience in the sales, marketing, and distribution of pharmaceutical products. There can be no assurance that the Company will be able to establish sales, marketing, and distribution capabilities or make arrangements through collaborations, licensees, or others to perform such activities, or that such efforts would be successful. If the Company decides to market its product directly, the Company must either acquire or internally develop a marketing and sales force with technical expertise and provide supporting distribution capabilities. The acquisition or development of a sales and distribution infrastructure would require substantial resources, which may divert the attention of management and key personnel, and have a negative impact on product development. If the Company contracts with third parties for the sales and marketing of its product, the Company’s revenue will be dependent on the efforts of these third parties, whose efforts may not be successful. If the Company fails to establish successful marketing and sales capabilities or to make arrangements with third parties, the business, financial condition and results of operations will be materially adversely affected.
Health Care Reimbursement
In both domestic and foreign markets, sales of the Company’s product, if any, will be dependent in part on the availability of reimbursement from third party payors, such as government and private insurance plans. Third party payors are increasingly challenging the prices charged for medical products and services. There can be no assurance that the Company’s product will be considered cost effective by these third party payors, that reimbursement will be available or if available that the payor’s reimbursement policies will not adversely affect the Company’s ability to sell its product on a profitable basis.
Government Regulation
The production and marketing of the Company’s product and its ongoing research and development activities are subject to regulation by numerous federal, provincial, state and local governmental authorities in Canada, the United States and any other countries where the Company may test or market its product. These laws require the approval of manufacturing facilities, including adhering to “good manufacturing” and/or “good laboratory” practices during production and storage, the controlled research and testing of products, governmental review and approval of submissions requiring manufacturing, pre-clinical and clinical data to establish the safety and efficacy of the product for each use sought in order to obtain marketing approval, and the control of marketing activities, including advertising and labeling. The process of obtaining required approvals (such as, but not limited to, the approval of the FDA in the United States, the European Medicines Agency and Health Canada) can be costly and time consuming and there can be no assurance that future products will be successfully developed, proven safe and effective in clinical trials or receive applicable regulatory approvals. Potential investors should be aware of the risks, problems, delays, expenses and difficulties which may be encountered by the Company in view of the extensive regulatory environment which controls its business.
17
In addition, there can be no assurance that the Company will be able to achieve or maintain regulatory compliance with respect to all or any part of its current or future products or that the Company will be able to timely and profitably produce its product while complying with applicable regulatory requirements. If the Company fails to maintain compliance, regulatory authorities may not allow the continuation of the drug development programs, or require the Company to make substantial changes to the drug. Any such actions could have a material adverse effect on the business, financial condition, and results of operations.
Unauthorized Disclosure of Confidential Information
There may be an unauthorized disclosure of the significant amount of confidential information under the Company’s control. The Company maintains and manages confidential information relating to its technology, research and development, production, marketing and business operations and those of its collaborators, in various forms. Although the Company has implemented controls to protect the confidentiality of such information, there can be no assurance that such controls will be effective. Unauthorized disclosures of such information could subject the Company to complaints or lawsuits for damages or could otherwise have a negative impact on its business, financial condition, results of operations, reputation and credibility.
Use of Hazardous Materials
Drug manufacturing processes involve the controlled use of hazardous materials. The Company and its third party manufacturing contractors are subject to regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although the Company believes that its third party manufacturers have the required safety procedures for handling and disposing of such materials and comply with the standards prescribed by such laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, the Company could be held liable for any damages that result and such liability could exceed the Company’s resources.
Risks Relating to the Offering
There is currently no market through which the Company’s Securities, other than the Common Shares, may be sold.
Unless otherwise specified in an applicable Prospectus Supplement, the Company’s Warrants and Subscription Receipts will not be listed on any securities or stock exchanges or on any automated dealer quotation system. There is currently no market through which the Company’s Securities, other than the Common Shares, may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of the Company’s Securities, other than the Common Shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these Securities and the extent of issuer regulation.
Volatility of Share Price
The market prices for the securities of biotechnology companies, including the Company, have historically been volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of any particular company. For example, since January 1, 2015, the closing price of the Company’s Common Shares on the TSX has ranged from a low of CDN$3.74 to a high of CDN$6.65 and the closing price of the Common Shares on NASDAQ has ranged from a low of US$2.79 to a high of US$5.30.
The trading price of the Company’s Common Shares could continue to be subject to wide fluctuations in price in response to various factors, many of which are beyond the Company’s control, including the results and adequacy of the Company’s preclinical studies and clinical trials, as well as those of its collaborators, or its competitors; other evidence of the safety or effectiveness of the Company’s product or those of its competitors; announcements of technological innovations or new products by the Company or its competitors; governmental regulatory actions; developments with collaborators; developments (including litigation) concerning patent or other proprietary rights of the Company or competitors; concern as to the safety of the Company’s product;
18
period-to-period fluctuations in operating results; changes in estimates of the Company’s performance by securities analysts; market conditions for biotechnology stocks in general; and other factors not within the control of the Company could have a significant adverse impact on the market price of the Company’s Securities, regardless of its operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A class action suit against the Company could result in substantial costs, potential liabilities and the diversion of management’s attention and resources.
There is no guarantee that an active trading market for the Company’s Common Shares will be maintained on the TSX and /or NASDAQ. Investors may not be able to sell their shares quickly or at the latest market price if the trading in the Company’s Common Shares is not active.
The Company expects to issue Common Shares in the future. Holders of stock options and warrants may elect to exercise their options or warrants into Common Shares depending on the stock price. Future issuances of Common Shares, or the perception that such issuances are likely to occur, could affect the prevailing trading prices of the Common Shares. Future issuances of the Company’s Common Shares could result in substantial dilution to its shareholders. In addition, the existence of Warrants may encourage short selling by market participants.
Sales of Common Shares could cause a decline in the market price of the Company’s Common Shares. Two of the Company’s major shareholders (venBio and ILJIN) own an aggregate of approximately 30% of the Company’s outstanding Common Shares as at October 16, 2015. Any sales of Common Shares by these shareholders or other existing shareholders or holders of options may have an adverse effect on the Company’s ability to raise capital and may adversely affect the market price of its Common Shares.
Future issuances of equity securities by the Company or sales by the Company’s existing shareholders may cause the price of the Common Shares to fall.
The market price of the Common Shares could decline as a result of issuances of Securities by the Company or sales by the Company’s existing shareholders in the market, or the perception that these sales could occur, during the currency of this Prospectus. Sales of Common Shares by shareholders might also make it more difficult for the Company to sell Common Shares at a time and price that the Company deems appropriate. With an additional sale or issuance of Common Shares, investors will suffer dilution of their voting power and may experience dilution in earnings per share.
The Company will have broad discretion in the use of the net proceeds of an offering of the Securities and may not use them to effectively manage the Company’s business.
The Company will have broad discretion over the use of the net proceeds from an offering of Securities. Because of the number and variability of factors that will determine the Company’s use of such proceeds, the Company’s ultimate use might vary substantially from the Company’s planned use. Investors may not agree with how the Company allocates or spends the proceeds from an offering of Common Shares. The Company may pursue acquisitions, collaborations or clinical trials that do not result in an increase in the market value of the Common Shares, and may increase the Company’s losses.
The Company does not intend to pay dividends in the foreseeable future.
The Company has never declared or paid any dividends on the Common Shares. The Company intends, for the foreseeable future, to retain its future earnings, if any, to finance its commercial activities and further research and the expansion of its business. As a result, the return on an investment in Common Shares will likely depend upon any future appreciation in value, if any, and on a shareholder’s ability to sell Common Shares. The payment of future dividends, if any, will be reviewed periodically by the Company’s board of directors and will depend upon, among other things, conditions then existing including earnings, financial conditions, cash on hand, financial requirements to fund the Company’s commercial activities, development and growth, and other factors that the Company’s board of directors may consider appropriate in the circumstances.
19
The Company may be a passive foreign investment company for U.S. tax purposes, which may result in adverse tax consequences for U.S. investors.
Investors in Common Shares that are U.S. taxpayers should be aware that the Company believes that it was not, for the financial year ended December 31, 2014, a “passive foreign investment company” under Section 1297(a) of the U.S. Internal Revenue Code (a “PFIC”). However, there is no certainty that taxation authorities in the U.S. would agree with the Company’s determination, and there is no certainty that the Company will not be a PFIC at some point in the future. If the Company is determined to be or becomes a PFIC, generally any gain recognized on the sale of the Common Shares and any “excess distributions” (as specially defined) paid on the Common Shares must be ratably allocated to each day in a U.S. taxpayer’s holding period for the Common Shares. The amount of any such gain or excess distribution allocated to prior years of such U.S taxpayer’s holding period for the Common Shares generally will be subject to U.S federal income tax at the highest tax applicable to ordinary income in each such prior year, and the U.S. taxpayer will be required to pay interest on the resulting tax liability for each such prior year, calculated as if such tax liability had been due in each such prior year.
Alternatively, a U.S. taxpayer that makes a “qualified electing fund” (a “QEF”) election with respect to the Company generally will be subject to U.S. federal income tax on such U.S. taxpayer’s pro rata share of the Company’s “net capital gain” and “ordinary earnings” (as specifically defined and calculated under U.S. federal income tax rules), regardless of whether such amounts are actually distributed by the Company. U.S. taxpayers should be aware that there can be no assurance that the Company will satisfy record keeping requirements under the QEF rules or that the Company will supply U.S. taxpayers with required information under the QEF rules, in the event that the Company is a PFIC and a U.S. taxpayer wishes to make a QEF election. As a second alternative, a U.S. taxpayer may make a “mark-to-market election” if the Company is a PFIC and the Common Shares are “marketable stock” (as specifically defined). A U.S. taxpayer that makes a mark-to-market election generally will include in gross income, for each taxable year in which the Company is a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the Common Shares as of the close of such taxable year over (b) such U.S. taxpayer’s adjusted tax basis in the Common Shares.
The above paragraphs contain only a brief summary of certain U.S. federal income tax considerations. Investors should consult their own tax advisor regarding the PFIC rules and other U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares.
You may be unable to enforce actions against the Company, certain of the Company’s directors and officers, or the experts named in this Prospectus under U.S. federal securities laws.
The Company is a corporation organized under the laws of Alberta, Canada. Most of the Company’s directors and officers, as well as the experts named in this Prospectus, reside principally in Canada or outside of the United States. Because all or a substantial portion of the Company’s assets and the assets of these persons are located outside of the United States, it may not be possible for investors to effect service of process within the United States upon the Company or those persons. Furthermore, it may not be possible for investors to enforce against the Company or those persons in the United States, judgments obtained in U.S. courts based upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of the U.S. federal securities laws. Therefore, it may not be possible to enforce those actions against the Company, certain of the Company’s directors and officers or the experts named in this Prospectus.
There have been no material changes in the share and loan capital of the Company, on a consolidated basis, since the date of the most recently filed unaudited financial statements of the Company.
20
Unless the Company otherwise indicates in a Prospectus Supplement, the Company currently intends to use the net proceeds from the sale of the Securities for working capital and other general corporate purposes, which includes, but is not limited to, clinical development, regulatory and pre-marketing activities for voclosporin primarily for LN but also potentially for other voclosporin indications and business development opportunities such as additional product in-licensing transactions. The clinical trial development for voclosporin includes conducting the required Phase 3 clinical program for LN. It is expected that more than 10% of the net proceeds from any distribution under this Prospectus will be used for research and development. Accordingly, each Prospectus Supplement will include a description of the timing and stage of research and development programs that will be funded by such proceeds, the major components of such programs (included anticipated costs), a statement of whether the Company is conducting its own research and development, subcontracting for those services or a combination thereof, and the additional steps required to reach commercial production and an estimate of costs and timing. There may be circumstances where, on the basis of results obtained or for other sound business reasons, a re-allocation of funds may be necessary or prudent. Accordingly, management of the Company will have broad discretion in the application of the proceeds of an offering of Securities. The Company’s ultimate use might vary substantially from what is stated in this Prospectus or a Prospectus Supplement and the actual amount that the Company spends in connection with each intended use of proceeds may vary significantly from the amounts specified in the applicable Prospectus Supplement and will depend on a number of factors, including those referred to under “Risk Factors” and any other factors set forth in the applicable Prospectus Supplement.
The use of proceeds allocated to working capital will be used to fund corporate, administration and business development activities in support of the research and development activities undertaken by the Company.
The Company had negative cash flow from operating activities for the year ended December 31, 2014 as it commenced its Phase 2b trial for LN during 2014 and expects that the proceeds from any distribution under this Prospectus will primarily be used to fund expected negative cash flow from operating activities as the Company continues with its voclosporin clinical development program.
More detailed information regarding the use of proceeds from the sale of Securities will be described in any applicable Prospectus Supplement.
The applicable Prospectus Supplement will describe prior sales of the Company as required in a Prospectus Supplement with respect to the issuance of Securities pursuant to such Prospectus Supplement.
The Common Shares are listed on the TSX in Canada (trading symbol: AUP) and on NASDAQ in the United States (trading symbol: AUPH). The Common Shares began trading on the NASDAQ on September 2, 2014.
21
The following table sets forth, for the periods indicated, the reported high and low prices (in Canadian dollars) and volume of Common Shares traded for each month on the TSX.
TSX
| Month | Price Range (CDN$) | Total Volume | ||||||||||
| High | Low | |||||||||||
| October, 2014 |
$ | 4.35 | $ | 2.75 | 237,664 | |||||||
| November, 2014 |
$ | 4.39 | $ | 3.26 | 212,501 | |||||||
| December, 2014 |
$ | 4.50 | $ | 3.88 | 131,237 | |||||||
| January, 2015 |
$ | 4.44 | $ | 4.00 | 626,833 | |||||||
| February, 2015 |
$ | 5.90 | $ | 3.86 | 557,449 | |||||||
| March, 2015 |
$ | 7.00 | $ | 5.11 | 394,125 | |||||||
| April, 2015 |
$ | 5.60 | $ | 4.42 | 194,198 | |||||||
| May, 2015 |
$ | 5.12 | $ | 4.18 | 147,577 | |||||||
| June, 2015 |
$ | 4.50 | $ | 3.70 | 107,458 | |||||||
| July, 2015 |
$ | 4.94 | $ | 3.80 | 316,426 | |||||||
| August, 2015 |
$ | 5.34 | $ | 3.51 | 148,626 | |||||||
| September 2015 |
$ | 4.66 | $ | 3.75 | 44,178 | |||||||
| October 1 to 15, 2015(1) |
$ | 4.09 | $ | 3.69 | 24,574 | |||||||
| (1) | October 15, 2015 was the last trading day prior to the date of this Prospectus. |
The following table sets forth, for the periods indicated, the reported high and low prices (in United States dollars) and the volume of shares traded for each month on NASDAQ.
NASDAQ
| Month | Price Range (US$) |
Total Volume | ||||||||||
| High | Low | |||||||||||
| October, 2014 |
$ | 4.01 | $ | 1.41 | 180,204 | |||||||
| November, 2014 |
$ | 4.35 | $ | 3.20 | 286,137 | |||||||
| December, 2014 |
$ | 5.39 | $ | 3.50 | 331,640 | |||||||
| January, 2015 |
$ | 3.96 | $ | 3.08 | 523,951 | |||||||
| February, 2015 |
$ | 4.86 | $ | 3.03 | 769,165 | |||||||
| March, 2015 |
$ | 5.65 | $ | 4.11 | 2,895,790 | |||||||
| April, 2015 |
$ | 4.52 | $ | 3.66 | 1,096,718 | |||||||
| May, 2015 |
$ | 4.37 | $ | 3.44 | 1,049,840 | |||||||
| June, 2015 |
$ | 3.60 | $ | 2.99 | 662,465 | |||||||
| July, 2015 |
$ | 3.78 | $ | 3.00 | 2,455,759 | |||||||
| August, 2015 |
$ | 4.30 | $ | 2.91 | 961,414 | |||||||
| September 2015 |
$ | 3.59 | $ | 2.78 | 545,100 | |||||||
| October 1 to 15, 2015(1) |
$ | 3.34 | $ | 2.84 | 130,133 | |||||||
| (1) | October 15, 2015 was the last trading day prior to the date of this Prospectus. |
The Company is authorized to issue an unlimited number of Common Shares, without nominal or par value. As of October 16, 2015, 32,287,419 Common Shares are issued and outstanding. In addition, 5,916,114 Common Shares are reserved for issuance upon exercise of existing warrants and 2,670,192 Common Shares have been reserved for issuance pursuant to outstanding options.
22
The holders of Common Shares are entitled to one (1) vote per share held at meetings of shareholders, to receive such dividends as declared by the Company and to receive a share of the remaining property and assets of the Company upon dissolution or winding up of the Company. The Common Shares are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such shares.
The Company may issue Warrants to purchase Common Shares. The Company may issue Warrants independently or together with other Securities, and Warrants sold with other Securities may be attached to or separate from the other Securities. Warrants will be issued under and governed by the terms of one or more warrant indentures (each a “Warrant Indenture”) between the Company and a warrant trustee (the “Warrant Trustee”) that the Company will name in the relevant Prospectus Supplement. Each Warrant Trustee will be a financial institution or trust company organized under the laws of Canada or any province thereof and authorized to carry on business as a trustee.
This summary of some of the provisions of the Warrants is not complete. The statements made in this Prospectus relating to any Warrant Indenture and Warrants to be issued under this Prospectus are summaries of certain anticipated provisions thereof and do not purport to be complete and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Warrant Indenture. Prospective investors should refer to the Warrant Indenture relating to the specific Warrants being offered for the complete terms of the Warrants. A copy of the form of Warrant Indenture will be filed by the Company with the applicable securities regulatory authorities in Canada and the United States.
The applicable Prospectus Supplement relating to any Warrants offered by the Company will describe the particular terms of those Warrants and include specific terms relating to the offering. This description will include, where applicable:
| • | the designation and aggregate number of Warrants; |
| • | the price at which the Warrants will be offered; |
| • | the currency or currencies in which the Warrants will be offered; |
| • | the date on which the right to exercise the Warrants will commence and the date on which the right will expire; |
| • | the number of Common Shares that may be purchased upon exercise of each Warrant and the price at which and currency or currencies in which the Common Shares may be purchased upon exercise of each Warrant; |
| • | the designation and terms of any Securities with which the Warrants will be offered, if any, and the number of the Warrants that will be offered with each Security; |
| • | the date or dates, if any, on or after which the Warrants and the other Securities with which the Warrants will be offered will be transferable separately; |
| • | whether the Warrants will be subject to redemption and, if so, the terms of such redemption provisions; |
| • | whether the Company will issue the Warrants as global securities and, if so, the identity of the depositary of the global securities; |
| • | whether the Warrants will be listed on any exchange; |
| • | material United States and Canadian federal income tax consequences of owning the Warrants; and |
| • | any other material terms or conditions of the Warrants. |
23
Rights of Holders Prior to Exercise
Prior to the exercise of their Warrants, holders of Warrants will not have any of the rights of holders of the Common Shares issuable upon exercise of the Warrants.
Global Securities
The Company may issue Warrants in whole or in part in the form of one or more global securities, which will be registered in the name of and be deposited with a depositary, or its nominee, each of which will be identified in the applicable Prospectus Supplement. The global securities may be in temporary or permanent form. The applicable Prospectus Supplement will describe the terms of any depositary arrangement and the rights and limitations of owners of beneficial interests in any global security. The applicable Prospectus Supplement also will describe the exchange, registration and transfer rights relating to any global security.
Modifications
The Warrant Indenture will provide for modifications and alterations to the Warrants issued thereunder by way of a resolution of holders of Warrants at a meeting of such holders or a consent in writing from such holders. The number of holders of Warrants required to pass such a resolution or execute such a written consent will be specified in the Warrant Indenture.
The Company may amend any Warrant Indenture and the Warrants, without the consent of the holders of the Warrants, to cure any ambiguity, to cure, correct or supplement any defective or inconsistent provision, or in any other manner that will not materially and adversely affect the interests of holders of outstanding Warrants.
DESCRIPTION OF SUBSCRIPTION RECEIPTS
The Company may issue Subscription Receipts, which will entitle holders to receive upon satisfaction of certain release conditions and for no additional consideration, Common Shares, Warrants or any combination thereof. Subscription Receipts will be issued pursuant to one or more subscription receipt agreements (each, a “Subscription Receipt Agreement”), each to be entered into between the Company and an Escrow Agent (the “Escrow Agent”), which will establish the terms and conditions of the Subscription Receipts. Each Escrow Agent will be a financial institution or trust company organized under the laws of Canada or a province thereof and authorized to carry on business as a trustee. A copy of the form of Subscription Receipt Agreement will be filed by the Company with the applicable securities regulatory authorities in Canada and the United States.
The following description sets forth certain general terms and provisions of Subscription Receipts and is not intended to be complete. The statements made in this Prospectus relating to any Subscription Receipt Agreement and Subscription Receipts to be issued thereunder are summaries of certain anticipated provisions thereof and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Subscription Receipt Agreement and the Prospectus Supplement describing such Subscription Receipt Agreement.
The Prospectus Supplement relating to any Subscription Receipts the Company offers will describe the Subscription Receipts and include specific terms relating to their offering. All such terms will comply with the requirements of the TSX and NASDAQ relating to Subscription Receipts. If underwriters or agents are used in the sale of Subscription Receipts, one or more of such underwriters or agents may also be parties to the Subscription Receipt Agreement governing the Subscription Receipts sold to or through such underwriters or agents.
24
General
The Prospectus Supplement and the Subscription Receipt Agreement for any Subscription Receipts the Company offers will describe the specific terms of the Subscription Receipts and may include, but are not limited to, any of the following:
| • | the designation and aggregate number of Subscription Receipts offered; |
| • | the price at which the Subscription Receipts will be offered; |
| • | the currency or currencies in which the Subscription Receipts will be offered; |
| • | the designation, number and terms of the Common Shares, Warrants or combination thereof to be received by holders of Subscription Receipts upon satisfaction of the release conditions, and the procedures that will result in the adjustment of those numbers; |
| • | the conditions (the “Release Conditions”) that must be met in order for holders of Subscription Receipts to receive for no additional consideration Common Shares, Warrants or a combination thereof; |
| • | the procedures for the issuance and delivery of Common Shares, Warrants or a combination thereof to holders of Subscription Receipts upon satisfaction of the Release Conditions; |
| • | whether any payments will be made to holders of Subscription Receipts upon delivery of the Common Shares, Warrants or a combination thereof upon satisfaction of the Release Conditions (e.g., an amount equal to dividends declared on Common Shares by the Company to holders of record during the period from the date of issuance of the Subscription Receipts to the date of issuance of any Common Shares pursuant to the terms of the Subscription Receipt Agreement); |
| • | the terms and conditions under which the Escrow Agent will hold all or a portion of the gross proceeds from the sale of Subscription Receipts, together with interest and income earned thereon (collectively, the “Escrowed Funds”), pending satisfaction of the Release Conditions; |
| • | the terms and conditions pursuant to which the Escrow Agent will hold Common Shares, Warrants or a combination thereof pending satisfaction of the Release Conditions; |
| • | the terms and conditions under which the Escrow Agent will release all or a portion of the Escrowed Funds to the Company upon satisfaction of the Release Conditions; |
| • | if the Subscription Receipts are sold to or through underwriters or agents, the terms and conditions under which the Escrow Agent will release a portion of the Escrowed Funds to such underwriters or agents in payment of all or a portion of their fees or commission in connection with the sale of the Subscription Receipts; |
| • | procedures for the refund by the Escrow Agent to holders of Subscription Receipts of all or a portion of the subscription price for their Subscription Receipts, plus any pro rata entitlement to interest earned or income generated on such amount, if the Release Conditions are not satisfied; |
| • | any contractual right of rescission to be granted to initial purchasers of Subscription Receipts in the event this Prospectus, the Prospectus Supplement under which Subscription Receipts are issued or any amendment hereto or thereto contains a misrepresentation; |
| • | any entitlement of the Company to purchase the Subscription Receipts in the open market by private agreement or otherwise; |
| • | whether the Company will issue the Subscription Receipts as global securities and, if so, the identity of the depositary for the global securities; |
| • | whether the Company will issue the Subscription Receipts as bearer securities, registered securities or both; |
25
| • | provisions as to modification, amendment or variation of the Subscription Receipt Agreement or any rights or terms attaching to the Subscription Receipts; |
| • | the identity of the Escrow Agent; |
| • | whether the Subscription Receipts will be listed on any exchange; |
| • | material United States and Canadian federal tax consequences of owning the Subscription Receipts; and |
| • | any other terms of the Subscription Receipts. |
The holders of Subscription Receipts will not be shareholders of the Company. Holders of Subscription Receipts are entitled only to receive Common Shares, Warrants or a combination thereof on exchange of their Subscription Receipts, plus any cash payments provided for under the Subscription Receipt Agreement, if the Release Conditions are satisfied. If the Release Conditions are not satisfied, Holders of Subscription Receipts shall be entitled to a refund of all or a portion of the subscription price therefor and all or a portion of the pro rata share of interest earned or income generated thereon, as provided in the Subscription Receipt Agreement.
Escrow
The Escrowed Funds will be held in escrow by the Escrow Agent, and such Escrowed Funds will be released to the Company (and, if the Subscription Receipts are sold to or through underwriters or agents, a portion of the Escrowed Funds may be released to such underwriters or agents in payment of all or a portion of their fees in connection with the sale of the Subscription Receipts) at the time and under the terms specified by the Subscription Receipt Agreement. If the Release Conditions are not satisfied, holders of Subscription Receipts will receive a refund of all or a portion of the subscription price for their Subscription Receipts plus their pro-rata entitlement to interest earned or income generated on such amount, in accordance with the terms of the Subscription Receipt Agreement. Common Shares or Warrants may be held in escrow by the Escrow Agent, and will be released to the holders of Subscription Receipts following satisfaction of the Release Conditions at the time and under the terms specified in the Subscription Receipt Agreement.
Rescission
The Subscription Receipt Agreement will also provide that any misrepresentation in this Prospectus, the Prospectus Supplement under which the Subscription Receipts are offered, or any amendment thereto, will entitle each initial purchaser of Subscription Receipts to a contractual right of rescission following the issuance of the Common Shares or Warrants to such purchaser entitling such purchaser to receive the amount paid for the Subscription Receipts upon surrender of the Common Shares or Warrants, provided that such remedy for rescission is exercised in the time stipulated in the Subscription Receipt Agreement. This right of rescission does not extend to holders of Subscription Receipts who acquire such Subscription Receipts from an initial purchaser, on the open market or otherwise, or to initial purchasers who acquire Subscription Receipts in the United States.
Global Securities
The Company may issue Subscription Receipts in whole or in part in the form of one or more global securities, which will be registered in the name of and be deposited with a depositary, or its nominee, each of which will be identified in the applicable Prospectus Supplement. The global securities may be in temporary or permanent form. The applicable Prospectus Supplement will describe the terms of any depositary arrangement and the rights and limitations of owners of beneficial interests in any global security. The applicable Prospectus Supplement also will describe the exchange, registration and transfer rights relating to any global security.
26
Modifications
The Subscription Receipt Agreement will provide for modifications and alterations to the Subscription Receipts issued thereunder by way of a resolution of holders of Subscription Receipts at a meeting of such holders or a consent in writing from such holders. The number of holders of Subscriptions Receipts required to pass such a resolution or execute such a written consent will be specified in the Subscription Receipt Agreement.
Common Shares may be sold under this Prospectus by way of a secondary offering by or for the account of certain of the Company’s securityholders. The Prospectus Supplement that the Company will file in connection with any offering of Common Shares by selling securityholders will include the following information:
| • | the names of the selling securityholders; |
| • | the number or amount of Common Shares owned, controlled or directed by each selling securityholder; |
| • | the number or amount of Common Shares being distributed for the account of each selling securityholder; |
| • | the number or amount of securities of the Company to be owned by the selling securityholders after the distribution and the percentage that number or amount represents of the total number of the Company’s outstanding securities; |
| • | whether Common Shares are owned by the selling securityholders both of record and beneficially, of record only or beneficially only; |
| • | if the selling securityholder purchased the Common Shares being distributed within two years preceding the date of the Prospectus Supplement, the date or dates the selling securityholder acquired the Common Shares; and |
| • | if the selling securityholder acquired the Common Shares being distributed in the twelve months preceding the date of the Prospectus Supplement, the cost thereof to the selling securityholder in the aggregate and on a per share basis. |
New Issue
The Company may sell the Securities offered by this Prospectus:
| • | to or through underwriters, dealers, placement agents or other intermediaries; |
| • | directly to one or more purchasers, or |
| • | in connection with acquisitions by the Company. |
The applicable Prospectus Supplement will set forth the terms of the offering of the Securities, including:
| • | the name or names of any underwriters, dealers or other placement agents; |
| • | the purchase price of, and form of consideration for, the Securities and the proceeds to the Company; |
| • | any delayed delivery arrangements; |
| • | any underwriting commissions, fees, discounts, and other items constituting underwriters’ compensation; |
| • | any offering price; |
27
| • | any discounts or concessions allowed or re-allowed or paid to dealers; and |
| • | any securities exchanges on which the Securities may be listed. |
Only the underwriters named in a Prospectus Supplement are deemed to be underwriters in connection with the Securities offered by that Prospectus Supplement.
The Securities may be sold, from time to time in one or more transactions at a fixed price or prices which may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing market price or at negotiated prices, including sales in transactions that are deemed to be “at the market distributions” as defined in National Instrument 44-102 – Shelf Distributions.
Under agreements which may be entered into by the Company, underwriters, dealers and agents who participate in the distribution of Securities may be entitled to indemnification by the Company against certain liabilities, including liabilities under any applicable Canadian provincial securities legislation, or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof. The underwriters, dealers and agents with whom the Company enters into agreements may be customers of, engage in transactions with, or perform services for, the Company in the ordinary course of business.
In connection with the offering of the Securities, the underwriters may over-allot or effect transactions which stabilize or maintain the market price of the Securities offered at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. A purchaser that acquires Securities forming part of the underwriters’ over-allocation position acquires those Securities under this Prospectus, regardless of whether the over-allocation position is ultimately filled through the exercise of the over-allotment option or secondary market purchases. No underwriter or dealer involved in the distribution, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot Securities or effect any other transactions that are intended to stabilize or maintain the market price of the Securities in connection with any distribution of Securities that is an “at the market distribution.”
Secondary Offering
This Prospectus may also, from time to time, relate to the offering of Common Shares by certain selling securityholders.
The selling securityholders may sell all or a portion of Common Shares beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If Common Shares are sold through underwriters or broker-dealers, the selling securityholders will be responsible for underwriting discounts or commissions or agent’s commissions. Common Shares may be sold by the selling securityholders in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions, as follows:
| • | on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| • | in the over-the-counter market; |
| • | in transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| • | through the writing of options, whether such options are listed on an options exchange or otherwise; |
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
28
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | short sales; |
| • | sales pursuant to Rule 144 under the U.S. Securities Act; |
| • | broker-dealers may agree with the selling securityholders to sell a specified number of such shares at a stipulated price per share; |
| • | a combination of any such methods of sale; and |
| • | any other method permitted pursuant to applicable law. |
If the selling securityholders effect such transactions by selling Common Shares to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling securityholders or commissions from purchasers of Common Shares for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of Common Shares or otherwise, the selling securityholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of Common Shares in the course of hedging in positions they assume. The selling securityholders may also sell Common Shares short and deliver Common Shares covered by this Prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling securityholders may also loan or pledge Common Shares to broker-dealers that in turn may sell such shares.
The selling securityholders may pledge or grant a security interest in some or all of the Common Shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell Common Shares from time to time pursuant to this Prospectus or any Prospectus Supplement filed under General Instruction II.L. of Form F-10 under the U.S. Securities Act, amending, if necessary, the list of selling securityholders to include, pursuant to a prospectus amendment or Prospectus Supplement, the pledgee, transferee or other successors in interest as selling securityholders under this Prospectus. The selling securityholders also may transfer and donate Common Shares in other circumstances in which case the transferees, donees, pledgees or other successor in interest will be the selling beneficial owners for purposes of this Prospectus.
The selling securityholders and any broker-dealer participating in the distribution of Common Shares may be deemed to be “underwriters” within the meaning of the U.S. Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the U.S. Securities Act. At the time a particular offering of Common Shares is made, a Prospectus Supplement, if required, will be distributed which will identify the selling securityholders and provide the other information set forth under “Selling Securityholders”, set forth the aggregate amount of Common Shares being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling securityholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
Under the securities laws of some states, Common Shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states Common Shares may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any securityholder will sell any or all of Common Shares registered pursuant to the registration statement, of which this prospectus forms a part.
29
The selling securityholders and any other person participating in such distribution will be subject to applicable provisions of Canadian securities legislation and the U.S. Exchange Act and the rules and regulations thereunder, including, without limitations, Regulation M under the U.S. Exchange Act, which may limit the timing of purchases and sales of any of the Company’s Common Shares by the selling securityholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the Company’s Common Shares to engage in market-making activities with respect to the Company’s Common Shares. All of the foregoing may affect the marketability of the Company’s Common Shares and the ability of any person or entity to engage in market-making activities with respect to the Company’s Common Shares.
Once sold under the shelf registration statement, of which this Prospectus forms a part, Common Shares will be freely tradable in the hands of persons other than the Company’s affiliates.
CERTAIN INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain Canadian federal income tax consequences to an investor who is a non-resident of Canada or to an investor who is a resident of Canada of acquiring, owning or disposing of any of the Company’s Securities offered thereunder.
The applicable Prospectus Supplement may also describe certain U.S. federal income tax consequences of the acquisition, ownership and disposition of any of the Company’s Securities, offered thereunder by an initial investor who is a U.S. person (within the meaning of the U.S. Internal Revenue Code), including, to the extent applicable, such consequences related to debt securities payable in a currency other than the U.S. dollar, issued at an original issue discount for U.S. federal income tax purposes or containing early redemption provisions or other special items.
The auditors of Aurinia Pharmaceuticals Inc. are PricewaterhouseCoopers LLP, Edmonton, Alberta, Canada. PricewaterhouseCoopers LLP has reported on the Company’s fiscal 2013 and 2014 audited consolidated financial statements, which have been filed with the securities regulatory authorities and incorporated herein. PricewaterhouseCoopers LLP is independent with respect to the Company within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of Alberta.
TRANSFER AGENTS AND REGISTRARS
The co-transfer agents and co-registrars of Aurinia Pharmaceuticals Inc. are Computershare Investor Services Inc. located at its principal offices in Calgary, Alberta and Toronto, Ontario and Computershare Trust Company, N.A. located at its principal offices in Golden, Colorado.
30
Gregory Ayers, Hyuek Joon Lee, David Jayne, Charles Rowland and Stephen Zaruby are directors of the Company and reside outside of Canada. Each of these directors has appointed the following agent for service of process in Canada:
| Name of Person |
Name and Address of Agent | |
| Gregory Ayers, Hyuek Joon Lee, David Jayne, Charles Rowland and Stephen Zaruby | Borden Ladner Gervais LLP 1200 Waterfront Centre 200 Burrard Street, P.O. Box 48600 Vancouver, BC V7X 1T2 | |
| Attention: Stephen P. Robertson |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
31
Certain legal matters relating to the Securities offered by this Prospectus will be passed upon for the Company by Borden Ladner Gervais LLP, Vancouver, British Columbia. The partners and associates of Borden Ladner Gervais LLP, Vancouver, British Columbia beneficially own, directly or indirectly, less than 1% of the Common Shares issued by Aurinia Pharmaceuticals Inc.
WHERE CAN YOU FIND MORE INFORMATION
The Company is required to file with the securities commission or authority in each of the applicable provinces of Canada annual and quarterly reports, material change reports and other information. In addition, the Company is subject to the informational requirements of the Exchange Act, and, in accordance with the Exchange Act, the Company also files reports with, and furnishes other information to, the SEC. Under a multijurisdictional disclosure system adopted by the United States and Canada, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ in certain respects from those in the United States. As a foreign private issuer, the Company is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Company’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, the Company is not required to publish financial statements as promptly as U.S. companies.
You may read any document the Company files with or furnishes to the securities commissions and authorities of the applicable provinces of Canada through SEDAR and any document the Company files with, or furnishes to, the SEC at the SEC’s public reference room at Station Place, 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms. Certain of the Company’s filings are also electronically available on EDGAR, and may be accessed at www.sec.gov.
ENFORCEABILITY OF CIVIL LIABILITIES
The Company is a corporation existing under the Business Corporations Act (Alberta). Most of the Company’s directors and officers, and the experts named in this Prospectus, are residents of Canada or otherwise reside outside the United States, and all or a substantial portion of their assets may be, and a substantial portion of the Corporation’s assets are, located outside the United States. The Company has appointed an agent for service of process in the United States (as set forth below) but it may be difficult for holders of securities who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon the Company’s civil liability and the civil liability of the Company’s directors, officers and experts under the United States federal securities laws. The Company has been advised that a judgment of a U.S. court predicated solely upon civil liability under U.S. federal securities laws or the securities of “blue sky” laws of any state within the United States, would likely be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. The Company has also been advised, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of the liability predicated solely upon U.S. federal securities laws.
The Company filed with the SEC, concurrently with the Company’s registration statement on Form F-10 of which this Prospectus is a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, the Company appointed CT Corporation System, 111 Eighth Avenue, New York, New York 10011 as the Company’s agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving the Company in a U.S. court arising out of or related to or concerning the offering of securities under this Prospectus.
32
CANADIAN PURCHASER’S STATUTORY AND CONTRACTUAL RIGHTS
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a Prospectus or a Prospectus Supplement relating to the securities purchased by a purchaser and any amendments thereto. In several of the provinces, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revision of the price or damages if the Prospectus or a Prospectus Supplement relating to the securities purchased by a purchaser and any amendments thereto contain a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for the particulars of these rights or consult with a legal adviser.
In an offering of Warrants or Subscription Receipts, investors are cautioned that the statutory right of action for damages for a misrepresentation contained in the Prospectus is limited, in certain provincial securities legislation, to the price at which the Warrants or Subscription Receipts are offered to the public under the Prospectus offering. This means that, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon conversion or exchange of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal adviser.
Original purchasers of Warrants or Subscription Receipts (if offered separately) will have a contractual right of rescission against Aurinia in respect of the exercise of such Warrant or Subscription Receipt. The contractual right of rescission will entitle such original purchasers to receive, in addition to the amount paid on original purchase of the Warrant or Subscription Receipt the amount paid upon exercise upon surrender of the underlying securities gained thereby, in the event that this Prospectus (as supplemented or amended) contains a misrepresentation, provided that: (i) the exercise takes place within 180 days of the date of the purchase of the Warrant or Subscription Receipt under this Prospectus; and (ii) the right of rescission is exercised within 180 days of the date of purchase of the Warrant or Subscription Receipt under this Prospectus. This contractual right of rescission will be consistent with the statutory right of rescission described under section 131 of the Securities Act (British Columbia), and is in addition to any other right or remedy available to original purchasers under section 131 of the Securities Act (British Columbia) or otherwise at law.
Original purchasers are further advised that in certain provinces the statutory right of action for damages in connection with a prospectus misrepresentation is limited to the amount paid for the security that was purchased under a prospectus, and therefore a further payment at the time of exercise may not be recoverable in a statutory action for damages. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights, or consult with a legal advisor.
33
CERTIFICATE OF AURINIA PHARMACEUTICALS INC.
Dated: October 16, 2015
This short form prospectus, together with the documents incorporated in this prospectus by reference, will, as of the date of the last supplement to this prospectus relating to the securities offered by this prospectus and the supplement(s), constitute full, true and plain disclosure of all material facts relating to the securities offered by this prospectus and the supplement(s) as required by the securities legislation of British Columbia, Alberta and Ontario.
| /s/ STEPHEN W. ZARUBY |
/s/ DENNIS BOURGEAULT | |
| Stephen W. Zaruby | Dennis Bourgeault | |
| President and Chief Executive Officer | Chief Financial Officer |
On Behalf of the Board of Directors
| /s/ RICHARD GLICKMAN |
/s/ CHARLES A. ROWLAND, JR. | |
| Richard Glickman | Charles A. Rowland, Jr. | |
| Director | Director |
34
11,111,111 Units

Prospectus Supplement
, 2016
Sole Book-Running Manager
H.C. Wainwright & Co.
Co-Manager
Cormark Securities Inc.