
Filed Pursuant to General Instruction II.L. of Form F-10
File No. 333-222413
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise.
This prospectus supplement, together with the short form base shelf prospectus dated March 26, 2018 to which it relates, as amended or supplemented, and each document incorporated or deemed to be incorporated by reference in the short form base shelf prospectus, constitutes a public offering of securities offered pursuant hereto only in the jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities. See “Plan of Distribution.”
Information has been incorporated by reference in this prospectus supplement from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of Aurinia Pharmaceuticals Inc. at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia, Canada, V7X 1T2, telephone (604) 632-3473, and are also available electronically at www.sec.gov/edgar.shtml or www.sedar.com.
PROSPECTUS SUPPLEMENT No. 1
To the Short Form Base Shelf Prospectus Dated March 26, 2018
| Secondary Offering | April 5, 2018 |

AURINIA PHARMACEUTICALS INC.
4,492,098 Common Shares
This prospectus supplement relates to the periodic resale of common shares (“Common Shares”) in the capital of Aurinia Pharmaceuticals Inc. (“Aurinia,” the “Company,” “we,” “us” or “our”) by the holders of certain of our common share purchase warrants (each a “Warrantholder”) during the period that the accompanying short form base shelf prospectus dated March 26, 2018, including any amendments thereto, remains valid (the “Offering”). In connection with (i) the sale of securities (the “June 2016 Private Placement”) on a private placement basis to certain of the Warrantholders and (ii) the sale of securities (the “December 2016 Public Offering”) under a public offering to certain of the Warrantholders, this prospectus supplement covers resales by the Warrantholders, from time to time, of up to 968,432 Common Shares issuable on the exercise of common share purchase warrants exercisable for a period of two years from the date of issuance at an exercise price of US$2.77 (a “June 2016 Warrant”) and up to 3,523,666 Common Shares issuable on the exercise of common share purchase warrants exercisable for a period of five years from the date of issuance at an exercise price of US$3.00 (a “December 2016 Warrant”) (collectively, the “Registrable Common Shares”). See “Plan of Distribution”. In connection with the June 2016 Private Placement, we entered into a registration rights agreement dated June 22, 2016 (the “Registration Rights Agreement”) with certain Warrantholders. We agreed in the Registration Rights Agreement to bear all fees and expenses incident to the registration of the Registrable Common Shares, other than fees and disbursements of counsel to the applicable Warrantholders. The Registrable Common Shares were previously registered pursuant to a prior short form base shelf prospectus which has since expired.
No underwriter has been involved in the preparation of, or has performed any review of, this prospectus supplement.
Our Common Shares are listed and posted for trading on the Toronto Stock Exchange (the “TSX”) under the symbol “AUP” and on the Nasdaq Global Market (the “Nasdaq”) under the symbol “AUPH.” On April 4, 2018, the closing price of the Common Shares on the TSX was CDN$6.74 and the closing price of the Common Shares on the Nasdaq was US$5.29.
Investing in our securities involves a high degree of risk. You should carefully read the “Risk Factors” section in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein, as well as the information under the heading “Cautionary Note Regarding Forward Looking Information” in this prospectus supplement, and consider such notes and information in connection with an investment in any securities.
The Warrantholders may, from time to time, sell, transfer or otherwise dispose of any or all of the Registrable Common Shares or interests in the Registrable Common Shares on any stock exchange, market or trading facility on which the Registrable Common Shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices. Prices may vary from purchaser to purchaser during the period of distribution. See “Plan of Distribution”. This prospectus supplement has not been filed in respect of, and will not qualify, any distribution of the Registrable Common Shares in British Columbia or in any other province or territory of Canada at any time. We will not receive any of the proceeds from the sale or other disposition of the Registrable Common Shares by the Warrantholders. The net proceeds received from the sale or other disposition of the Registrable Common Shares by the Warrantholders, if any, is unknown.
Investors should rely only on current information contained in or incorporated by reference into this prospectus supplement as such information is accurate only as of the date of the applicable document. We have not authorized anyone to provide investors with different information. Information contained on our website shall not be deemed to be a part of this prospectus supplement or incorporated by reference and should not be relied upon by prospective investors for the purpose of determining whether to invest in the securities. These securities are not being offered in any jurisdiction where the offer or sale is not permitted. Investors should not assume that the information contained in this prospectus supplement is accurate as of any date other than the date on the face page of this prospectus supplement or the date of any documents incorporated by reference herein.
We are permitted under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United States to prepare this prospectus supplement and the accompanying prospectus in accordance with the disclosure requirements of Canada. Prospective investors in the United States should be aware that such requirements are different from those of the United States. The financial statements incorporated by reference in this prospectus supplement and the accompanying prospectus have been prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, and are subject to Canadian auditing and auditor independence standards. As a result, our financial statements may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the owning our securities may have tax consequences both in Canada and the United States. Such consequences, for investors who are resident in, or citizens of, the United States, may not be described fully in this prospectus supplement, including the Canadian federal income tax consequences applicable to a foreign controlled Canadian corporation that acquires Common Shares. Investors should read the tax discussion in this prospectus supplement and the accompanying prospectus and consult their own tax advisors with respect to their own particular circumstances. See the sections titled “Certain Canadian Federal Income Tax Considerations,” “Material U.S. Federal Income Taxation Considerations” and “Risk Factors.”
Your ability to enforce civil liabilities under the United States federal securities laws may be affected adversely because we are incorporated in Canada, most of the officers and directors and some of the experts named in this prospectus supplement are not residents of the United States, and many of our assets and all or a substantial portion of the assets of such persons are located outside of the United States. See “Enforceability of Certain Civil Liabilities.”
Neither the U.S. Securities and Exchange Commission (the “SEC”) nor any state or Canadian securities regulator has approved or disapproved the securities offered hereby; passed upon the accuracy or adequacy of this prospectus supplement or determined if this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense.
Our registered office is located at #201, 17904 – 105 Avenue, Edmonton, Alberta T5S 2H5, Canada. Our head office is located at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8, Canada.
PROSPECTUS SUPPLEMENT
| S-1 | ||||
| S-1 | ||||
| S-2 | ||||
| S-5 | ||||
| S-7 | ||||
| S-7 | ||||
| S-7 | ||||
| S-7 | ||||
| S-11 | ||||
| S-11 | ||||
| S-11 | ||||
| S-12 | ||||
| S-13 | ||||
| S-14 | ||||
| S-14 | ||||
| S-14 | ||||
| S-19 | ||||
| S-23 | ||||
| S-25 | ||||
| S-25 | ||||
| S-26 | ||||
| S-26 | ||||
| S-26 | ||||
| S-26 | ||||
| C-1 | ||||
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of the securities that are being offered pursuant to this prospectus supplement and the method of distribution of those securities and also supplements and updates information regarding us contained in the accompanying prospectus. The second part, the accompanying prospectus, gives more general information about securities we may offer from time to time, some of which may not apply to the securities offered pursuant to this prospectus supplement. Both documents contain important information you should consider when making your investment decision. This prospectus supplement may add, update or change information contained in the accompanying prospectus. Before investing, you should carefully read both this prospectus supplement and the accompanying prospectus together with the additional information about us to which we refer you in the sections of this prospectus supplement titled “Documents Incorporated by Reference” and “Where You Can Find More Information.”
You should rely only on information contained in this prospectus supplement, the accompanying prospectus and the documents we incorporate by reference in this prospectus supplement and the accompanying prospectus. If information in this prospectus supplement is inconsistent with the accompanying prospectus or the information incorporated by reference, you should rely on this prospectus supplement. We have not authorized anyone to provide you with information that is different. If anyone provides you with any different or inconsistent information, you should not rely on it. The Common Shares will be offered only in jurisdictions where such offers are permitted by law. This prospectus supplement has not been filed in respect of, and will not qualify, any distribution of the Registrable Common Shares in British Columbia or in any other province or territory of Canada at any time. The information contained in this prospectus supplement and the accompanying prospectus is accurate only as of their respective dates, regardless of the time of delivery of this prospectus supplement and the accompanying prospectus and you should not assume otherwise.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference into this prospectus supplement and the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is part of a “shelf” registration statement on Form F-10 that we filed with the SEC. The shelf registration statement was declared effective by the SEC on March 29, 2018. This prospectus supplement does not contain all of the information contained in the registration statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC. You should refer to the registration statement and the exhibits to the registration statement for further information with respect to us and our securities.
In this prospectus supplement, unless stated otherwise or the context requires, all dollar amounts are expressed in U.S. dollars. All references to “$” or “US$” are to the lawful currency of the United States and all references to “CDN$” are to the lawful currency of Canada. This prospectus supplement and the documents incorporated by reference contain translations of some Canadian dollar amounts into U.S. dollars solely for your convenience. See the section titled “Exchange Rate Information.”
Market data and certain industry forecasts used in this prospectus supplement and the documents incorporated by reference herein or therein were obtained from market research, publicly available information and industry publications. We believe that these sources are generally reliable, but the accuracy and completeness of this information is not guaranteed. We have not independently verified such information, and we do not make any representation as to the accuracy of such information.
S-1
In this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein, unless the context otherwise requires, references to “we,” “us,” “our” or similar terms, as well as references to “Aurinia” or the “Company,” refer to Aurinia Pharmaceuticals Inc., together with our subsidiaries.
This prospectus supplement is deemed to be incorporated by reference into the accompanying prospectus solely in connection with the Registrable Common Shares. Other documents are also incorporated or deemed to be incorporated by reference into this prospectus supplement and into the accompanying prospectus. See the section titled “Documents Incorporated by Reference.”
This prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein contain “forward-looking statements” or “forward-looking information” within the meaning of applicable securities legislation.
A statement is forward-looking when it uses what we know and expect today to make a statement about the future. Forward-looking statements may include words such as “anticipate”, “believe”, “intend”, “expect”, “goal”, “may”, “outlook”, “plan”, “seek”, “project”, “should”, “strive”, “target”, “could”, “continue”, “potential” and “estimated”, or the negative of such terms or comparable terminology. You should not place undue reliance on the forward-looking statements, particularly those concerning anticipated events relating to the development, clinical trials, regulatory approval, and marketing of our product and the timing or magnitude of those events, as they are inherently risky and uncertain.
Securities laws encourage companies to disclose forward-looking information so that investors can get a better understanding our future prospects and make informed investment decisions. These statements, made either in this prospectus supplement or a document incorporated by reference in this prospectus supplement, may include, without limitation:
| • | our belief that the Phase IIb lupus nephritis AURA- LV (“AURA”) clinical trial had positive results; |
| • | our belief that we have sufficient cash resources to adequately fund operations through Phase III lupus nephritis (“AURORA”) clinical trial results and regulatory submission; |
| • | our belief that confirmatory data generated from the single AURORA clinical trial and the recently completed AURA clinical trial should support regulatory submissions in the United States, Europe and Japan and the timing of such, including the New Drug Application submission in the United States; |
| • | our belief that recently granted formulation patents regarding the delivery of voclosporin to the ocular surface for conditions such as dry eye have the potential to be of therapeutic value; |
| • | our plans and expectations and the timing of commencement, enrollment, completion and release of results of clinical trials; |
| • | our current forecast for the cost of the AURORA clinical trial and the continuation study; |
| • | our intention to seek regulatory approvals in the United States, Europe and Japan for voclosporin and anticipated timing of receiving approval; |
| • | our intention to demonstrate that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class status for the treatment of lupus nephritis (“LN”) outside of Japan; |
| • | our belief in voclosporin being potentially a best-in-class CNI (as defined below) with robust intellectual property exclusivity and the benefits over existing commercially available CNIs; |
| • | our belief that voclosporin has further potential to be effectively used across a range of therapeutic autoimmune areas including focal segmental glomerularsclerosis (“FSGS”) and dry eye syndrome (“DES”); |
S-2
| • | our intention to initiate a Phase II clinical trial for voclosporin in FSGS patients and the timing for commencement and for data availability for the same; |
| • | our intention to commence a Phase IIa tolerability study of voclosporin ophthalmic solution (“VOS”) and the timing for commencement and for data availability for the same; |
| • | statements concerning the anticipated commercial potential of voclosporin for the treatment of LN, FSGS and DES; |
| • | our belief that the expansion of the renal franchise could create significant value for shareholders; |
| • | our intention to use the net proceeds from financings for various purposes; |
| • | our belief that Aurinia’s current financial resources are sufficient to fund all existing programs, the new indication expansion and new product development work and supporting operations into 2020. |
| • | our plans to generate future revenues from products licensed to pharmaceutical and biotechnology companies |
| • | statements concerning partnership activities and health regulatory discussions; |
| • | statements concerning the potential market for voclosporin; |
| • | our ability to take advantage of financing opportunities if and when needed; |
| • | our belief that VOS has the potential to compete in the multi-billion-dollar human prescription dry eye market; |
| • | our intention to seek additional corporate alliances and collaborative agreements to support the commercialization and development of our product; and |
| • | our belief that the annualized pricing for voclosporin could range between $45,000-$100,000 in the United States. |
Such statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based on a number of estimates and assumptions that, while considered reasonable by management, as at the date of such statements, are inherently subject to significant business, economic, competitive, political, regulatory, legal, scientific and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The factors and assumptions used by management to develop such forward-looking statements include, but are not limited to:
| • | the assumption that we will be able to obtain approval from regulatory agencies on executable development programs with parameters that are satisfactory to us; |
| • | the assumption that recruitment to clinical trials will occur as projected; |
| • | the assumption that we will successfully complete our clinical programs on a timely basis, including conducting the required AURORA clinical trial and meet regulatory requirements for approval of marketing authorization applications and new drug approvals, as well as favourable product labelling; |
| • | the assumption that the planned studies will achieve positive results; |
| • | the assumptions regarding the costs and expenses associated with Aurinia’s clinical trials; |
| • | the assumption the regulatory requirements and commitments will be maintained; |
| • | the assumption that we will be able to meet GMP standards and manufacture and secure a sufficient supply of voclosporin on a timely basis to successfully complete the development and commercialization of voclosporin; |
| • | the assumptions on the market value for the LN program; |
| • | the assumption that our patent portfolio is sufficient and valid; |
S-3
| • | the assumption that we will be able to extend our patents on terms acceptable to us; |
| • | the assumptions on the market; |
| • | the assumption that there is a potential commercial value for other indications for voclosporin; |
| • | the assumption that market data and reports reviewed by us are accurate; |
| • | the assumption that another company will not create a substantial competitive product for Aurinia’s LN business without violating Aurinia’s intellectual property rights; |
| • | the assumptions on the burn rate of Aurinia’s cash for operations; |
| • | the assumption that our current good relationships with our suppliers, service providers and other third parties will be maintained; |
| • | the assumption that we will be able to attract and retain a sufficient amount of skilled staff and/or |
| • | the assumptions relating to the capital required to fund operations through AURORA clinical trial results and regulatory submission. |
It is important to know that:
| • | actual results could be materially different from what we expect if known or unknown risks affect our business, or if our estimates or assumptions turn out to be inaccurate. As a result, we cannot guarantee that any forward-looking statement will materialize and, accordingly, you are cautioned not to place undue reliance on these forward-looking statements. |
| • | forward-looking statements do not take into account the effect that transactions or non-recurring or other special items announced or occurring after the statements are made may have on our business. For example, they do not include the effect of mergers, acquisitions, other business combinations or transactions, dispositions, sales of assets, asset write-downs or other charges announced or occurring after the forward-looking statements are made. The financial impact of such transactions and non-recurring and other special items can be complex and necessarily depends on the facts particular to each of them. Accordingly, the expected impact cannot be meaningfully described in the abstract or presented in the same manner as known risks affecting our business. |
The factors discussed below and other considerations discussed in the “Risk Factors” section of this prospectus supplement could cause our actual results to differ significantly from those contained in any forward-looking statements.
Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to differ materially from any assumptions, further results, performance or achievements expressed or implied by such forward-looking statements. Important factors that could cause such differences include, among other things, the following:
| • | the need for additional capital in the longer term to fund our development programs and the effect of capital market conditions and other factors on capital availability; |
| • | competition in our marketplace; |
| • | difficulties, delays, or failures we may experience in the conduct of and reporting of results of our clinical trials for voclosporin; |
| • | difficulties in meeting Good Manufacturing Practice (“GMP”) standards and the manufacturing and securing a sufficient supply of voclosporin on a timely basis to successfully complete the development and commercialization of voclosporin; |
| • | difficulties, delays or failures in obtaining regulatory approvals for the initiation of clinical trials; |
S-4
| • | difficulties in gaining alignment among the key regulatory jurisdictions, European Medicines Agency (“EMA”), Food and Drug Administration (“FDA”) and Pharmaceutical and Medical Devices Agency (“PMDA”), which may require further clinical activities; |
| • | difficulties, delays or failures in obtaining regulatory approvals to market voclosporin; |
| • | not being able to extend our patent portfolio for voclosporin; |
| • | difficulties we may experience in completing the development and commercialization of voclosporin; |
| • | the market for the LN business may not be as we have estimated; |
| • | insufficient acceptance of and demand for voclosporin; |
| • | difficulties obtaining adequate reimbursements from third party payors; |
| • | difficulties obtaining formulary acceptance; |
| • | competitors may arise with similar products; |
| • | product liability, patent infringement and other civil litigation; |
| • | injunction, court orders, regulatory and other enforcement actions; |
| • | we may have to pay unanticipated expenses, and/or estimated costs for clinical trials or operations may be underestimated, resulting in our having to make additional expenditures to achieve our current goals; |
| • | difficulties, restrictions, delays, or failures in obtaining appropriate reimbursement from payers for voclosporin; and/or |
| • | difficulties we may experience in identifying and successfully securing appropriate vendors to support the development and commercialization of our product. |
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These forward-looking statements are made as of the date of this prospectus supplement or, in the case of documents incorporated by reference in this prospectus supplement, as of the date of such documents and we disclaim any intention and have no obligation or responsibility, except as required by law, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this prospectus supplement from documents filed with securities commissions or similar authorities in Canada which have also been filed with, or furnished to, the SEC. Copies of the documents incorporated by reference in this prospectus supplement and not delivered with this prospectus supplement may be obtained on request without charge from our Corporate Secretary at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, Canada, Telephone: (604) 632-3473 or by accessing the disclosure documents through the Internet on the Canadian System for Electronic Analysis and Retrieval, or SEDAR, at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC’s Electronic Data Gathering and Retrieval System, or EDGAR, at www.sec.gov.
The following documents, filed with the securities commissions or similar regulatory authorities in British Columbia, Alberta and Ontario, and filed with, or furnished to, the SEC are specifically incorporated by reference into, and form an integral part of, this prospectus supplement:
| (a) | our annual information form dated March 13, 2018 for the fiscal year ended December 31, 2017; |
S-5
| (b) | our audited consolidated financial balance sheets as at December 31, 2017 and 2016 and the consolidated statements of operations and comprehensive loss, changes in shareholders’ equity (deficit) and cash flows for the two-year period ended December 31, 2017, including notes thereto and the auditors’ report thereon, as filed on March 15, 2018; |
| (c) | our management’s discussion and analysis of financial condition and results of operations for the year ended December 31, 2017; and |
| (d) | our management information circular dated May 12, 2017 in connection with the annual general meeting of our shareholders on June 21, 2017. |
Any documents of the type described in Section 11.1 of Form 44-101F1 Short Form Prospectuses that we have filed with a securities commission or similar authority in any jurisdiction of Canada subsequent to the date of this prospectus supplement and during the period that this prospectus supplement is effective will be deemed to be incorporated by reference into this prospectus supplement.
In addition, to the extent that any document or information incorporated by reference into this prospectus supplement is filed with, or furnished to, the SEC pursuant to the Securities Exchange Act of 1934, as amended (the “Exchange Act”), after the date of this prospectus supplement, that document or information will be deemed to be incorporated by reference as an exhibit to the registration statement of which this prospectus supplement forms a part (in the case of a report on Form 6-K, if and to the extent expressly provided therein).
Any statement contained in this prospectus supplement or in a document incorporated or deemed to be incorporated by reference in this prospectus supplement shall be deemed to be modified or superseded, for the purposes of this prospectus supplement, to the extent that a statement contained herein or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement.
Upon filing a new annual information form and the related annual financial statements and management’s discussion and analysis with applicable securities regulatory authorities during the currency of this prospectus supplement, the previous annual information form, the previous annual financial statements and management’s discussion and analysis and all quarterly financial statements, supplemental information and material change reports filed prior to the commencement of our financial year in which the new annual information form is filed will be deemed no longer to be incorporated into this prospectus supplement for purposes of future offers and sales of the Securities under this prospectus supplement. Upon interim consolidated financial statements and the accompanying management’s discussion and analysis being filed by us with the applicable securities regulatory authorities during the duration of this prospectus supplement, all interim consolidated financial statements and the accompanying management’s discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer to be incorporated into this prospectus supplement for purposes of future offers and sales of Securities under this prospectus supplement. Upon a new management information circular in connection with an annual meeting of shareholders being filed by us with the applicable securities regulatory authorities during the duration of this prospectus supplement, the management information circular filed in connection with the previous annual meeting of shareholders will be deemed no longer to be incorporated by reference in this prospectus supplement for purposes of future offers and sales of Securities under this prospectus supplement.
S-6
References to our website in any documents that are incorporated by reference into this prospectus supplement do not incorporate by reference the information on such website into this prospectus supplement or the accompanying prospectus, and we disclaim any such incorporation by reference.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
In addition to the documents specified in this prospectus supplement under the section titled “Documents Incorporated by Reference,” the following documents have been or will be (through post-effective amendment or incorporation by reference) filed with the SEC as part of the registration statement insofar as required by the SEC’s Form F-10: (i) powers of attorney from our directors and officers; and (ii) the consents of auditors and legal counsel.
The following description of Aurinia is derived from selected information about us contained in the documents incorporated by reference and does not contain all of the information about us and our business that should be considered before investing in the securities. This prospectus supplement may add to, update or change information in the accompanying prospectus. You should carefully read this entire prospectus supplement and the accompanying prospectus, including the risks and uncertainties discussed in the section titled “Risk Factors,” and the information incorporated by reference in this prospectus supplement, including our consolidated financial statements, before making an investment decision. If you invest in our securities, you are assuming a high degree of risk.
We are a clinical stage biopharmaceutical company with a head office at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8. Our registered office is located at #201, 17904-105 Avenue, Edmonton, Alberta T5S 2H5 where the finance function is performed.
We are organized under the Business Corporations Act (Alberta). The Common Shares are currently listed and traded on the Nasdaq under the symbol “AUPH” and on the TSX under the symbol “AUP”. Our primary business is the development of a therapeutic drug to treat autoimmune diseases, in particular LN.
We have the following wholly-owned subsidiaries: Aurinia Pharma U.S., Inc. (Delaware incorporated) and Aurinia Pharma Limited (UK incorporated).
Our By-Law No. 2 was amended at a shareholder’s meeting held on August 15, 2013 to include provisions requiring advance notice for any nominations of directors by shareholders, which are described further in our most recent information circular.
SUMMARY DESCRIPTION OF BUSINESS
We are focused on the development of our novel therapeutic immunomodulating drug candidate, voclosporin, for the treatment of LN, FSGS and DES. Voclosporin is a next generation calcineurin inhibitor (“CNI”) which has clinical data in over 2,400 patients across multiple indications. It has also been previously studied in kidney rejection following transplantation, psoriasis and in various forms of uveitis (an ophthalmic disease).
Legacy CNIs have demonstrated efficacy for a number of conditions, including LN, transplant, DES and other autoimmune diseases; however, side effects exist which can limit their long-term use and tolerability. Some clinical complications of legacy CNIs include hypertension, hyperlipidemia, diabetes, and both acute and chronic nephrotoxicity.
S-7
Voclosporin is an immunosuppressant, with a synergistic and dual mechanism of action that has the potential to improve near- and long-term outcomes in LN when added to mycophenolate mofetil (“MMF”), although not approved for such, the current standard of care for LN. By inhibiting calcineurin, voclosporin reduces cytokine activation and blocks interleukin IL-2 expression and T-cell mediated immune responses. Voclosporin also potentially stabilizes disease modifying podocytes, which protects against proteinuria. Voclosporin is made by a modification of a single amino acid of the cyclosporine molecule which has shown a more predictable pharmacokinetic and pharmacodynamic relationship, an increase in potency, an altered metabolic profile, and easier dosing without the need for therapeutic drug monitoring. Clinical doses of voclosporin studied to date range from 13 – 70 mg BID. The mechanism of action of voclosporin, a CNI, has been validated with certain first generation CNIs for the prevention of rejection in patients undergoing solid organ transplants and in several autoimmune indications, including dermatitis, keratoconjunctivitis sicca (“Dry Eye Syndrome” or “DES”), psoriasis, rheumatoid arthritis, and for LN in Japan.
We believe that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class regulatory approval status for the treatment of LN outside of Japan.
Based on published data, we believe the key potential benefits of voclosporin in the treatment of LN are as follows:
| • | increased potency compared to cyclosporine A, allowing lower dosing requirements and fewer off target effects; |
| • | limited inter and intra patient variability, allowing for easier dosing without the need for therapeutic drug monitoring; |
| • | less cholesterolemia and triglyceridemia than cyclosporine A; and |
| • | limited incidence of glucose intolerance and diabetes at therapeutic doses compared to tacrolimus. |
Lupus Nephritis
LN is an inflammation of the kidney caused by systemic lupus erythematosus (“SLE”) and represents a serious manifestation of SLE. SLE is a chronic, complex and often disabling disorder. SLE is highly heterogeneous, affecting a wide range of organs and tissue systems. Unlike SLE, LN has straightforward disease measures (readily assessable and easily identified by specialty treaters) where an early response correlates with long-term outcomes, measured by proteinuria. In patients with LN, renal damage results in proteinuria and/or hematuria and a decrease in renal function as evidenced by reduced estimated glomerular filtration rate (“eGFR”), and increased serum creatinine levels. eGFR is assessed through the Chronic Kidney Disease Epidemiology Collaboration equation. Rapid control and reduction of proteinuria in LN patients measured at 6 months shows a reduction in the need for dialysis at 10 years (Chen et al., Clin J. Am Soc Neph., 2008). LN can be debilitating and costly and if poorly controlled, can lead to permanent and irreversible tissue damage within the kidney. Recent literature suggests severe LN progresses to end-stage renal disease (“ESRD”), within 15 years of diagnosis in 10%-30% of patients, thus making LN a serious and potentially life-threatening condition. SLE patients with renal damage have a 14-fold increased risk of premature death, while SLE patients with ESRD have a greater than 60-fold increased risk of premature death. Mean annual cost for patients (both direct and indirect) with SLE (with no nephritis) have been estimated to exceed $20,000 per patient, while the mean annual cost for patients (both direct and indirect) with LN who progress to intermittent ESRD have been estimated to exceed $60,000 per patient (Carls et al., JOEM., Volume 51, No. 1, January 2009).
FSGS
FSGS is a lesion characterized by persistent scarring identified by biopsy and proteinuria. FSGS is a cause of Nephrotic Syndrome (“NS”) and is characterized by high morbidity. NS is a collection of symptoms that indicate kidney damage, including: large amounts of protein in the urine; low levels of albumin and higher than normal fat and cholesterol levels in the blood, and edema. Similar to LN, early clinical response and reduction of proteinuria is thought to be critical to long-term kidney health and outcomes.
S-8
FSGS is likely the most common primary glomerulopathy and the most common primary glomerulopathy leading to ESRD. The incidence of FSGS and ESRD due to FSGS are increasing as time goes on. Precise estimates of incidence and prevalence are difficult to determine. According to NephCure Kidney International, more than 5400 patients are diagnosed with FSGS every year; however, this is considered an underestimate because a limited number of biopsies are performed. The number of FSGS cases are rising more than any other cause of NS and the incidence of FSGS is increasing through disease awareness and improved diagnosis. FSGS occurs more frequently in adults than in children and is most prevalent in adults 45 years or older. FSGS is most common in people of African American and Asian descent. It has been shown that the control of proteinuria is important for long-term dialysis-free survival of these patients. Currently, there are no approved therapies for FSGS in the United States and Europe.
DES
DES, or keratoconjunctivitis sicca, is a chronic disease in which a lack of moisture and lubrication on the eye’s surface results in irritation and inflammation of the eye. DES is a multifactorial, heterogeneous disease estimated to affect greater than 20 million people in the United States (Market Scope, 2010 Comprehensive Report on The Global Dry Eye Products Market).
LN Standard of Care
While at Aspreva, certain members of Aurinia’s management team executed the ALMS study which established CellCept® as the current standard of care for treating LN. The ALMS study was published in 2009 in the Journal of the American Society of Nephrology and in 2011 in the New England Journal of Medicine.
The American College of Rheumatology recommends that intravenous cyclophosphamide or MMF/CellCept® be used as first-line immunosuppressive therapy for LN. Despite their use, the ALMS study showed that the vast majority of patients failed to achieve CR, and almost half failed to have a renal response at 24 weeks for both of these therapeutics. Based upon the results of the ALMS study, we believe that a better solution is needed to improve renal response rates for LN.
Despite CellCept® being the current SoC for the treatment of LN, it remains far from adequate with fewer than 20% of patients on therapy actually achieving disease remission after six months of therapy. Data suggests that a LN patient who does not achieve rapid disease remission upon treatment is more likely to experience renal failure or require dialysis at 10 years (Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ; the Collaborative Study Group. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3:46-53.). Therefore, it is critically important to achieve disease remission as quickly and as effectively as possible.
Based on available data from the AURA clinical trial, we believe that voclosporin has the potential to address the critical need for LN by controlling active disease rapidly, lowering the overall steroid burden, impacting extra-renal disease and doing so with a convenient oral twice-daily treatment regimen.
Market Potential and Commercial Considerations
Our target launch date for voclosporin as a treatment for LN is late 2020 or early 2021.
The global immunology market which covers autoimmune conditions including SLE is set to rise from $57.7 billion in 2015 to $75.4 billion by 2022 according to GBI Research with the United States accounting for half of the market. The growth in the market is driven by continued increase in prevalence and incidence of autoimmune conditions in addition to new market entrants that are targeting better outcomes. There is significant unmet need for new therapies specifically in SLE and LN.
S-9
We have conducted market research including claims database reviews (where available) and physician based research. Our physician research included approximately 900 rheumatologists and nephrologists across the United States, Europe and Japan to better define the potential market size, estimated pricing and treatment paradigms in those jurisdictions. Using the U.S. MarketScan® database (with approximately ~180,000,000 insured lives in the United States) there were 445,000 SLE patients in the database (between January 2006 and June 2016) based on specific SLE diagnosis codes. The National Institute of Diabetes and Digestive and Kidney Diseases estimates that up to 50% of people with SLE are diagnosed with LN. The Lupus Foundation of America estimates that 60% of people with lupus may develop kidney problems. Using claims database research and physician research, we believe the diagnosed range of LN patients to be approximately 125,000 to 200,000 in the United States and 150,000 to 215,000 for Europe and Japan combined. In the United States, Europe and Japan, one in five LN patients are thought to be undiagnosed due to referring physicians being inefficient and inaccurate in diagnosing the condition according to our research.
Similar to other autoimmune disorders, LN is a flaring and remitting disease. The destructive disease cycle people with LN go through is depicted below. The disease cycles from being in remission to being in flare, achieving partial remission and being back in remission. Treatment objectives between LN and other autoimmune diseases are remarkably similar. In other autoimmune conditions such as Multiple Sclerosis, Crohn’s, Rheumatoid Arthritis and SLE physicians goals are to induce/maintain a remission of disease, decrease frequency of hospital or ambulatory care visits and limit long term disability. In LN specifically, physicians are trying to avoid further kidney damage, dialysis, renal transplantation, and death. According to a physician survey, the frequency of LN flares amongst treated patients was approximately every 14 months across the United States and Europe. The ability to get patients into remission quickly correlates with better long-term kidney outcomes as noted above.
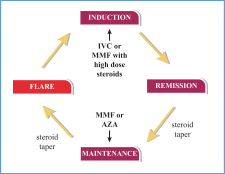
The population of people with LN will be in different cycles of their disease at any one time. Physicians currently use existing LN standard of care including immunosuppressants and high dose steroids to treat people with LN throughout the disease cycles including induction and maintenance phases. By studying voclosporin on top of an existing standard of care we are not seeking to displace current accepted treatment patterns. We feel that being additive to an existing standard of care in addition to the product being administered orally versus via infusion or injection can support a more rapid market adoption if approved.
Current annualized pricing (based on wholesale acquisition costs published by AnalySource® Reprinted with permission by First Databank, Inc. All rights reserved. © (2018)) for the treatments of other more prevalent autoimmune conditions such as Multiple Sclerosis, Crohn’s, Rheumatoid Arthritis and SLE ranges from $45,000-$100,000 in the United States. Wholesale acquisition cost is the manufacturer’s published catalog or list price for a drug product to wholesalers. We have conducted pricing research that studied a similar pricing range with payers and physicians and believe that pricing in this range can be achievable for voclosporin in the United States. Pricing for other autoimmune conditions is lower in Europe and Japan than it is in the United States driven by the specific country’s pricing and reimbursement processes. We expect that will be the case for voclosporin. We believe the United States will provide the largest market opportunity for the Company followed by Europe and Japan.
S-10
An investment in our securities is speculative and involves a high degree of risk. In addition to the other information included or incorporated by reference in this prospectus supplement and the accompanying prospectus, you should carefully consider the risks and uncertainties described under the heading “Risk Factors” in the accompanying prospectus, together with all of the other information contained in this prospectus supplement and the accompanying prospectus, before purchasing our securities. The occurrence of any of these risks could have a material adverse effect on our business, financial condition, results of operations and future prospects. In these circumstances, the market price of our Common Shares could decline, and you may lose all or part of your investment. These risks are not the only risks we face; risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business, financial condition and results of operations. Investors should also refer to the other information set forth or incorporated by reference in this prospectus supplement and the accompanying prospectus, including our consolidated financial statements and related notes. This prospectus supplement also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of a number of factors. See the section titled “Cautionary Note Regarding Forward-Looking Statements.”
CONSOLIDATED FINANCIAL INFORMATION AND CURRENCY
Our consolidated financial statements incorporated by reference in this prospectus supplement have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board, and are reported in United States dollars.
In this prospectus supplement, where applicable, and unless otherwise indicated, amounts are converted from Canadian dollars to U.S. dollars and vice versa by applying the closing rate of exchange for conversion of one Canadian dollar to U.S. dollars as reported by the Bank of Canada on April 4, 2018.
The following table sets forth for each period indicated: (i) the exchange rates in effect at the end of the period; (ii) the high and low exchange rates during such period; and (iii) the average exchange rates for such period, for one Canadian dollar, expressed in U.S. dollars, as quoted by the Bank of Canada.
| Year Ended December 31 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| US$ | US$ | US$ | ||||||||||
| Closing |
0.7971 | 0.7448 | 0.7225 | |||||||||
| High |
0.8245 | 0.7972 | 0.8527 | |||||||||
| Low |
0.7276 | 0.6854 | 0.7148 | |||||||||
| Average |
0.7708 | 0.7548 | 0.7820 | |||||||||
On April 4, 2018, the closing exchange rate as quoted by the Bank of Canada was CDN$1.00 = US$0.7807.
Since December 31, 2017, the date of our most recently filed annual audited financial statements, there have been no changes in our share capital on a consolidated basis other than as outlined under “Prior Sales.” For information on the exercise of options pursuant to our incentive stock option plan, and the exercise of certain outstanding warrants, see the section titled “Prior Sales.”
S-11
The Common Shares are listed on the TSX in Canada (trading symbol: AUP) and on Nasdaq in the United States (trading symbol: AUPH).
The following table sets forth, for the periods indicated, the reported high and low prices (in Canadian dollars) and volume of Common Shares traded for each month on the TSX.
TSX
| Price Range (CDN$) | Total Volume | |||||||||||
| Month |
High | Low | ||||||||||
| March, 2017 |
$ | 14.17 | $ | 4.80 | 12,279,153 | |||||||
| April, 2017 |
$ | 10.60 | $ | 8.65 | 2,649,488 | |||||||
| May, 2017 |
$ | 11.07 | $ | 7.75 | 2,062,784 | |||||||
| June, 2017 |
$ | 9.09 | $ | 7.81 | 962,381 | |||||||
| July, 2017 |
$ | 9.75 | $ | 7.80 | 1,418,472 | |||||||
| August, 2017 |
$ | 8.21 | $ | 7.20 | 711,171 | |||||||
| September, 2017 |
$ | 8.57 | $ | 7.50 | 978,676 | |||||||
| October, 2017 |
$ | 9.50 | $ | 7.04 | 1,613,476 | |||||||
| November, 2017 |
$ | 7.59 | $ | 6.00 | 1,693,411 | |||||||
| December, 2017 |
$ | 6.81 | $ | 5.68 | 917,741 | |||||||
| January, 2018 |
$ | 7.58 | $ | 5.70 | 1,344,258 | |||||||
| February, 2018 |
$ | 7.32 | $ | 5.98 | 854,513 | |||||||
| March, 2018 |
$ | 7.82 | $ | 6.49 | 973,583 | |||||||
| April 1 - 4, 2018(1) |
$ | 6.79 | $ | 6.44 | 70,786 | |||||||
| (1) | April 4, 2018 was the last trading day prior to the date of this prospectus supplement. |
The following table sets forth, for the periods indicated, the reported high and low prices (in United States dollars) and the volume of shares traded for each month on Nasdaq.
NASDAQ
| Price Range (US$) | Total Volume | |||||||||||
| Month |
High | Low | ||||||||||
| March, 2017 |
$ | 10.54 | $ | 3.58 | 421,107,989 | |||||||
| April, 2017 |
$ | 7.86 | $ | 6.42 | 102,463,110 | |||||||
| May, 2017 |
$ | 8.19 | $ | 5.74 | 74,595,039 | |||||||
| June, 2017 |
$ | 6.87 | $ | 5.89 | 34,543,620 | |||||||
| July, 2017 |
$ | 7.68 | $ | 6.17 | 35,665,295 | |||||||
| August, 2017 |
$ | 6.62 | $ | 5.66 | 21,374,588 | |||||||
| September, 2017 |
$ | 7.08 | $ | 6.10 | 19,202,130 | |||||||
| October, 2017 |
$ | 7.60 | $ | 5.54 | 39,120,821 | |||||||
| November, 2017 |
$ | 5.92 | $ | 4.68 | 17,378,314 | |||||||
| December, 2017 |
$ | 5.36 | $ | 4.41 | 21,669,860 | |||||||
| January, 2018 |
$ | 6.11 | $ | 4.52 | 22,658,500 | |||||||
| February, 2018 |
$ | 5.77 | $ | 4.76 | 12,473,400 | |||||||
| March , 2018 |
$ | 5.99 | $ | 5.05 | 14,836,900 | |||||||
| April 1 - April 4, 2018(1) |
$ | 5.29 | $ | 5.01 | 1,728,835 | |||||||
| (1) | April 4, 2018 was the last trading day prior to the date of this prospectus supplement. |
S-12
Common Shares
The following table summarizes details of the Common Shares we issued during the 12 month period prior to April 5, 2018.
| Month of Issuance |
Security |
Price per Security |
Number of Securities |
|||||
| April 6, 2017 |
Stock Options1 | CDN$3.91 | 1,500 | |||||
| April 10, 2017 |
Stock Options1 | US$3.50 | 110,000 | |||||
| April 13, 2017 |
Warrants2 | US$3.00 | 1,250 | |||||
| April 25, 2017 |
Stock Options1 | CDN$3.50 | 150,000 | |||||
| May 1, 2017 |
Warrants3 | US$ 3.00 | 748,805 | |||||
| May 8, 2017 |
Stock Options1 | US$3.50 | 10,000 | |||||
| May 9, 2017 |
Stock Options1 | CDN$3.50 | 150,000 | |||||
| May 18, 2017 |
Stock Options1 | US$3.50 | 150,000 | |||||
| May 19, 2017 |
Stock Options1 | CDN$3.91 | 417 | |||||
| May 19, 2017 |
Stock Options1 | CDN$4.21 | 222 | |||||
| May 25, 2017 |
Warrants2 | US$3.00 | 5,000 | |||||
| June 21, 2017 |
Stock Options1 | CDN$3.50 | 20,000 | |||||
| June 23, 2017 |
Stock Options1 | CDN$3.50 | 20,000 | |||||
| June 23, 2017 |
Stock Options1 | CDN$3.66 | 16,399 | |||||
| June 28, 2017 |
Stock Options1 | CDN$3.91 | 500 | |||||
| June 28, 2017 |
Stock Options1 | CDN$4.21 | 500 | |||||
| July 4, 2017 |
Warrants2 | US$2.77 | 7,415 | |||||
| July 25, 2017 |
Warrants4 | US$3.00 | 10,810 | |||||
| July 28, 2017 |
Stock Options1 | CDN$4.31 | 20,000 | |||||
| July 28 ,2017 |
Stock Options1 | CDN$3.96 | 10,000 | |||||
| July 28, 2017 |
Stock Options1 | CDN$4.73 | 1,667 | |||||
| September 12, 2017 |
Stock Options1 | CDN$3.20 | 437,444 | |||||
| October 5, 2017 |
Stock Options1 | CDN$3.50 | 20,000 | |||||
| October 19, 2017 |
Warrants5 | US$ 3.00 | 59,091 | |||||
|
|
|
|||||||
| Total |
1,951,020 | |||||||
|
|
|
|||||||
Note:
| 1 | Issued upon exercise of previously issued Stock Options. |
| 2 | Issued upon exercise of previously issued Warrants. |
| 3 | Issued pursuant to a cashless exercise provision. Calculation was determined by the number of warrants to be exercised (1,364,380) multiplied by a five day weighted average market price $US 7.14 less the exercise price ($US 3.2204) with the difference divided by the weighted average market price. |
| 4 | Issued pursuant to a cashless exercise provision. Calculation was determined by the number of warrants to be exercised (19,800) multiplied by a five day weighted average market price $US 7.09 less the exercise price ($US 3.2204) with the difference divided by the weighted average market price. |
| 5 | Issued pursuant to a cashless exercise provision. Calculation was determined by the number of warrants to be exercised (111,200) multiplied by a five day weighted average market price $US 6.94 less the exercise price ($US3.2204) with the difference divided by the weighted average market price. |
S-13
Stock Options
The following table summarizes details of the stock options we granted during the 12 month period prior to April 5, 2018.
| Month of Grant |
Security |
Grant Price per Security (CDN$) |
Number of Securities |
|||||
| April 26, 2017 |
Stock Options | 9.45 | 333,000 | |||||
| June 23, 2017 |
Stock Options | 8.48 | 50,000 | |||||
| July 5, 2017 |
Stock Options | 8.10 | 280,000 | |||||
| September 20, 2017 |
Stock Options | 7.59 | 60,000 | |||||
| October 25, 2017 |
Stock Options | 7.30 | 5,000 | |||||
| November 20, 2017 |
Stock Options | 6.56 | 30,000 | |||||
| February 1, 2018 |
Stock Options | 6.52 | 2,178,000 | |||||
| February 5, 2018 |
Stock Options | 6.42 | 550,000 | |||||
| February 9, 2018 |
Stock Options | 6.40 | 50,000 | |||||
| February 22, 2018 |
Stock Options | 6.92 | 50,000 | |||||
| March 21, 2018 |
Stock Options | 7.06 | 150,000 | |||||
|
|
|
|||||||
| Total |
3,736,000 | |||||||
|
|
|
|||||||
The proceeds from the sale or other disposition of the Registrable Common Shares covered by this prospectus supplement are solely for the account of the Warrant holders. Accordingly, we will not receive any proceeds from the sale or other disposition of the Registrable Common Shares by the Warrant holders. The net proceeds received from the sale or other disposition of the Registrable Common Shares by the Warrant holders, if any, is unknown.
Common Shares
We are authorized to issue an unlimited number of Common Shares, without nominal or par value. As of April 4, 2018, 84,051,758 Common Shares are issued and outstanding. In addition, 6,433,181 Common Shares are reserved for issuance upon exercise of existing warrants and 7,841,690 Common Shares have been reserved for issuance pursuant to outstanding options.
The holders of Common Shares are entitled to one (1) vote per share held at meetings of shareholders, to receive such dividends as declared by us and to receive a share of our remaining property and assets upon our dissolution or winding up. The Common Shares are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such shares.
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following discussion describes the material U.S. federal income tax consequences relating to the ownership and disposition of common shares by U.S. Holders (as defined below). This discussion applies to U.S. Holders that purchase common shares pursuant to the offering and hold such common shares as capital assets. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, all as in effect on the
S-14
date hereof and all of which are subject to change, possibly with retroactive effect. This discussion does not address all of the U.S. federal income tax consequences that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally mark their securities to market for U.S. federal income tax purposes, tax-exempt entities, retirement plans, regulated investment companies, real estate investment trusts, certain former citizens or residents of the United States, persons who hold common shares as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons subject to Section 451(b) of the Code, persons that have a “functional currency” other than the U.S. dollar, persons that own directly, indirectly or through attribution 10% or more of the voting power or value of our shares, corporations that accumulate earnings to avoid U.S. federal income tax, partnerships and other pass-through entities, and investors in such pass-through entities). This discussion does not address any U.S. state or local or non-U.S. tax consequences or any U.S. federal estate, gift or alternative minimum tax consequences.
As used in this discussion, the term “U.S. Holder” means a beneficial owner of common shares that is, for U.S. federal income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial decisions or (y) that has elected under applicable U.S. Treasury regulations to be treated as a domestic trust for U.S. federal income tax purposes.
If an entity treated as a partnership for U.S. federal income tax purposes holds common shares, the U.S. federal income tax consequences relating to an investment in the common shares will depend in part upon the status and activities of such entity and the particular partner. Any such entity should consult its own tax advisor regarding the U.S. federal income tax consequences applicable to it and its partners of the purchase, ownership and disposition of common shares.
Persons considering an investment in common shares should consult their own tax advisors as to the particular tax consequences applicable to them relating to the purchase, ownership and disposition of common shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Passive Foreign Investment Company Consequences
In general, a corporation organized outside the United States will be treated as a passive foreign investment company (a “PFIC”), for any taxable year in which either (1) at least 75% of its gross income is “passive income” (the “PFIC income test”), or (2) on average at least 50% of its assets, determined on a quarterly basis, are assets that produce passive income or are held for the production of passive income (the “PFIC asset test”). Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income generally include cash, even if held as working capital or raised in a public offering, marketable securities, and other assets that may produce passive income. Generally, in determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
We do not believe we were a PFIC for the year ended December 31, 2017. While we also do not believe we will be a PFIC for the current taxable year, because PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, there can be no assurance that we will not be a PFIC for the current taxable year. Because we hold a substantial amount of cash and cash equivalents, and because the calculation of the value of our assets may be based in part on the value of common shares, which may fluctuate considerably, we may also be a PFIC in future taxable years under the PFIC asset test. Even if we determine that
S-15
we are not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not successfully challenge our position. Our status as a PFIC is a fact-intensive determination made on an annual basis. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status and also expresses no opinion with regard to our expectations regarding our PFIC status.
If we are a PFIC in any taxable year during which a U.S. Holder owns common shares, the U.S. Holder could be liable for additional taxes and interest charges under the “PFIC excess distribution regime” upon (1) a distribution paid during a taxable year that is greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the common shares, and (2) any gain recognized on a sale, exchange or other disposition, including a pledge, of the common shares, whether or not we continue to be a PFIC. Under the PFIC excess distribution regime, the tax on such distribution or gain would be determined by allocating the distribution or gain ratably over the U.S. Holder’s holding period for common shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the current taxable year. The amount allocated to other taxable years will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable, to ordinary income for each such taxable year, and an interest charge, generally applicable to underpayments of tax, will be added to the tax.
If we are a PFIC for any year during which a U.S. Holder holds common shares, we must generally continue to be treated as a PFIC by that holder for all succeeding years during which the U.S. Holder holds the common shares, unless we cease to meet the requirements for PFIC status and the U.S. Holder makes a “deemed sale” election with respect to the common shares. If the election is made, the U.S. Holder will be deemed to sell the common shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime. After the deemed sale election, the U.S. Holder’s common shares would not be treated as shares of a PFIC unless we subsequently become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds common shares and one of our non-U.S. corporate subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions. Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries.
If we are a PFIC, a U.S. Holder will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on common shares if such U.S. Holder makes a valid “mark-to-market” election for our common shares. A mark-to-market election is available to a U.S. Holder only for “marketable stock.” Our common shares will be marketable stock as long as they remain listed on the NASDAQ and are regularly traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. If a mark-to-market election is in effect, a U.S. Holder generally would take into account, as ordinary income each year, the excess of the fair market value of common shares held at the end of such taxable year over the adjusted tax basis of such common shares. The U.S. Holder would also take into account, as an ordinary loss each year, the excess of the adjusted tax basis of such common shares over their fair market value at the end of the taxable year, but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the mark-to-market election. The U.S. Holder’s tax basis in common shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. Any gain from a sale, exchange or other disposition of common shares in any taxable year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated first as ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as capital loss.
S-16
A mark-to-market election will not apply to common shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any non-U.S. subsidiaries that we may organize or acquire in the future. Accordingly, a U.S. Holder may continue to be subject to tax under the PFIC excess distribution regime with respect to any lower-tier PFICs that we may organize or acquire in the future notwithstanding the U.S. Holder’s mark-to-market election for the common shares.
The tax consequences that would apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing fund (“QEF”) election. At this time we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make a QEF election, and therefore prospective investors should assume that a QEF election will not be available.
Each U.S. person that is an investor of a PFIC is generally required to file an annual information return on IRS Form 8621 containing such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
The U.S. federal income tax rules relating to PFICs are very complex. Prospective U.S. investors are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of common shares, the consequences to them of an investment in a PFIC, any elections available with respect to the common shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of common shares of a PFIC.
Distributions
Subject to the discussion above under “Passive Foreign Investment Company Consequences,” a U.S. Holder that receives a distribution with respect to common shares generally will be required to include the gross amount of such distribution (before reduction for any Canadian withholding taxes withheld therefrom) in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s common shares. To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s common shares, the remainder will be taxed as capital gain. Because we may not account for our earnings and profits in accordance with U.S. federal income tax principles, U.S. Holders should expect all distributions to be reported to them as dividends. Distributions on common shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute passive category income. Such dividends will not be eligible for the “dividends received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations.
Dividends paid by a “qualified foreign corporation” are eligible for taxation at a reduced capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that certain requirements are met. However, if we are a PFIC for the taxable year in which the dividend is paid or the preceding taxable year (see discussion above under “Passive Foreign Investment Company Consequences”), we will not be treated as a qualified foreign corporation, and therefore the reduced capital gains tax rate described above will not apply. Each U.S. Holder is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular circumstances.
A non-United States corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the
S-17
Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on common shares that are readily tradable on an established securities market in the United States. We believe that we qualify as a resident of Canada for purposes of, and are eligible for the benefits of, the U.S.-Canada Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Canada Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange of information provision. Therefore, subject to the discussion above under “Passive Foreign Investment Company Consequences,” if the U.S.-Canada Treaty is applicable, such dividends will generally be “qualified dividend income” in the hands of individual U.S. Holders, provided that certain conditions are met.
Sale, Exchange or Other Disposition of Common Shares
Subject to the discussion above under “Passive Foreign Investment Company Consequences,” a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of common shares in an amount equal to the difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received) on the sale, exchange or other disposition and such U.S. Holder’s adjusted tax basis in the common shares. Such capital gain or loss generally will be long-term capital gain taxable at a reduced rate for non-corporate U.S. Holders or long-term capital loss if, on the date of sale, exchange or other disposition, the common shares were held by the U.S. Holder for more than one year. Any capital gain of a non-corporate U.S. Holder that is not long-term capital gain is taxed at ordinary income rates. The deductibility of capital losses is subject to limitations. Any gain or loss recognized from the sale or other disposition of common shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of common shares. If you are a United States person that is an individual, estate or trust, you are encouraged to consult your tax advisors regarding the applicability of this Medicare tax to your income and gains in respect of your investment in common shares.
Information Reporting and Backup Withholding
U.S. Holders may be required to file certain U.S. information reporting returns with the IRS with respect to an investment in common shares, including, among others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment Company Consequences”, each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information. U.S. Holders paying more than US$100,000 for common shares may be required to file IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to comply with the required information reporting.
Dividends on and proceeds from the sale or other disposition of common shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if the holder (1) fails to provide an accurate United States taxpayer identification number or otherwise establish a basis for exemption, or (2) is described in certain other categories of persons. However, U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
U.S. Holders should consult their own tax advisors regarding the backup withholding tax and information reporting rules.
S-18
EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN COMMON SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
In this summary, an otherwise undefined term that first appears in quotation marks has the meaning ascribed to it in the Income Tax Act (Canada) (the “Tax Act”).
In the opinion of Borden Ladner Gervais LLP, Canadian legal counsel to Aurinia, the following fairly summarizes the principal Canadian federal income tax considerations under the Tax Act generally applicable as of this date to an investor who acquires Common Shares pursuant to the Offering and who, at all relevant times for the purposes of the Tax Act,
| • | deals at arm’s length with Aurinia, |
| • | is not affiliated with Aurinia, |
| • | holds all Common Shares as capital property, and |
is not, at any relevant time for those purposes,
| • | exempt from tax under Part I of the Tax Act, |
| • | a “financial institution” for the purposes of the “mark-to-market” property rules in the Tax Act, |
| • | a “specified financial institution,” |
| • | an entity or partnership an interest in which is a “tax shelter investment,” |
| • | a taxpayer who reports its “Canadian tax results” in a currency other than Canadian currency, or |
| • | a taxpayer, any of whose Common Shares will be the subject of a “derivative forward agreement,” “synthetic disposition agreement,” “synthetic equity agreement,” “synthetic equity arrangement,” or “specified synthetic equity arrangement,” |
(each such shareholder, in this summary, a “Holder”).
A Holder’s Common Shares will generally be considered to be capital property of the Holder provided that the Holder does not use the Common Shares in the course of carrying on a business of trading or dealing in securities, and has not acquired or been deemed to have acquired the Common Shares in one or more transactions considered to be an adventure or concern in the nature of trade. A Holder who is resident in Canada and whose Common Shares might not otherwise be capital property may, subject to certain restrictions and limitations in the Tax Act, be entitled to elect irrevocably pursuant to subsection 39(4) of the Tax Act that the Holder’s Common Shares, and every other “Canadian security” of the Holder, be capital property. Any Holder who is considering making a subsection 39(4) election should consult the Holder’s Canadian tax advisers before making the election.
This summary is based on the current provisions of the Tax Act and the Income Tax Regulations (Canada) (the “Regulations”) in force as of the date hereof, all specific proposals to amend the Tax Act or Regulations publicly announced by or on behalf of the Minister of Finance of Canada (“Finance”) on or before the date hereof, and counsel’s understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”). It is assumed that all such amendments will be enacted as currently proposed and that there will be no other change to the Tax Act, the Regulations, or the CRA’s administrative policies and assessing practices, although no assurance can be given in these respects. This summary does not otherwise take into account or anticipate any change in law or administrative policy or assessing practice whether by legislative,
S-19
governmental, or judicial decision or action, and does not take into account or consider any provincial, territorial or foreign income tax considerations, which may differ significantly from the Canadian federal income tax considerations discussed below.
Additional considerations, not discussed in this summary, may be applicable to a Holder that is a corporation resident in Canada, and is, or becomes, or does not deal at arm’s length for purposes of the Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or event or series of transactions or events that includes the acquisition of the Common Shares, controlled by a non-resident corporation for purposes of the “foreign affiliate dumping” rules in section 212.3 of the Tax Act. Such Holders should consult their Canadian tax advisers with respect to the consequences of acquiring Common Shares.
In addition, this summary does not address the deductibility of interest by a Holder that has borrowed money or otherwise incurred debt in connection with the acquisition of Common Shares. Such Holders should consult their Canadian tax advisers on this matter.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, and should not be construed to be, legal or tax advice to any particular Holder. Each Holder should consult the Holder’s own tax advisers with respect to the tax and legal consequences of acquiring, holding, and disposing of Common Shares applicable to the Holder’s particular circumstances.
Currency Conversion
Subject to certain exceptions that are not discussed in this summary, all amounts relevant to computing a Holder’s liability for tax (including dividends, adjusted cost base, and proceeds of disposition) under the Tax Act must, for the purposes of the Tax Act, be determined in Canadian dollars based on the rate quoted by the Bank of Canada for the applicable day or such other rate of exchange that is acceptable to the CRA. The amount of any dividend required to be included in a Holder’s income, or of any capital gain or capital loss realized by a Holder, may be affected by fluctuations in the Canadian dollar against other currencies.
Adjusted Cost Base
A Holder’s initial adjusted cost base of the Holder’s Common Shares acquired pursuant to this Offering will be determined by averaging the cost of those Common Shares with the Holder’s adjusted cost base of all Common Shares owned by the Holder as capital property immediately before the acquisition.
Resident Holders
The following section of this summary applies solely to Holders each of whom at all relevant times is or is deemed to be resident solely in Canada for the purposes of the Tax Act (each a “Resident Holder”).
On July 18, 2017, Finance released a consultation paper that included an announcement of the government’s intention to amend the Tax Act to increase the amount of tax applicable to certain investment income earned through a “private corporation” (the “July 2017 Tax Proposals”). The consultation period has ended and no specific amendments to the Tax Act have been proposed in connection with this announcement as of the date hereof. This summary does not address the potential implications of the July 2017 Tax Proposals. Holders that are private corporations should consult their tax advisors with respect to the implications of the July 2017 Tax Proposals as they relate to the acquisition, holding and disposition of Common Shares.
Dividends
A Resident Holder who is an individual (other than certain trusts) and receives or is deemed to receive a dividend on the Resident Holder’s Common Shares in a taxation year will generally be required to include the amount of
S-20
the dividend in income for the taxation year, subject to the gross-up and dividend tax credit rules applicable to a “taxable dividend” received from a “taxable Canadian corporation,” including the enhanced gross-up and dividend tax credit rules applicable to any dividend that Aurinia designates as an “eligible dividend” in accordance with the Tax Act.
A Resident Holder that is a corporation will generally be required to include the amount of any such dividend in its income for the taxation year, and entitled to deduct an equivalent amount from its taxable income for the year. In certain circumstances, subsection 55(2) of the Tax Act may deem some or all of the dividend to be a gain from the disposition of capital property rather than a dividend, in which case the rules described below under “Capital Gains and Capital Losses” would apply. Corporate Resident Holders should consult their own tax advisers regarding the potential application of subsection 55(2) to their particular circumstances.
A Resident Holder that is a “private corporation” or “subject corporation” may be subject to a refundable tax under Part IV of the Tax Act equal to 381/3% of the amount of the dividend to the extent that the dividend is deductible in computing the corporation’s taxable income. The tax generally will be refunded to the corporate Resident Holder at the rate of CDN$1.15 for each CDN$3.00 of taxable dividends that it pays while it is a private corporation.
Disposition of Common Shares
A Resident Holder who disposes or is deemed to dispose of a Common Share in a taxation year will generally realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of the Common Share, net of any reasonable costs of disposition, are greater (or less) than the Resident Holder’s adjusted cost base of the Common Shares determined immediately before the disposition. The tax treatment of capital gains and capital losses is discussed in greater detail below under the subheading “Capital Gains and Capital Losses.”
Capital Gains and Capital Losses
A Resident Holder who realizes or is deemed to realize a capital gain or capital loss in a taxation year on the disposition of a Common Share will generally be required to include one half of any such capital gain (a “taxable capital gain”) in income for the year, and entitled to deduct one half of any such capital loss (an “allowable capital loss”) from taxable capital gains realized by the Resident Holder in the year or, to the extent not so deductible, in any of the Resident Holder’s three preceding taxation years or any subsequent taxation year, subject to the detailed rules in the Tax Act regarding the deductibility of allowable capital losses.
The amount of any capital loss realized on the disposition or deemed disposition of a Common Share by a Resident Holder that is a corporation may be reduced by the amount of dividends that the Resident Holder received or is deemed to have received on the Common Share or a share substituted therefor, to the extent and in the circumstances specified by the Tax Act. Similar rules may apply to a Common Share owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary, as the case may be. Resident Holders to whom these rules may be relevant should consult their own tax advisers.
A Resident Holder that is throughout the relevant taxation year a “Canadian-controlled private corporation” may be liable to pay an additional refundable tax of 102/3% on certain investment income including taxable capital gains, and dividends or deemed dividends that are not deductible in computing taxable income. This refundable tax generally will be refunded to the corporate Resident Holder at the rate of CDN$1.15 for each CDN$3.00 of taxable dividends that it pays while it is a “private corporation.”
Minimum Tax
A Resident Holder who is an individual (including certain trusts) and realizes a capital gain or receives a dividend may thereby be subject to minimum tax under the Tax Act. Such Resident Holders should consult their own tax advisers in this regard.
S-21
Non-resident Holders
The following section of this summary is applicable solely to Holders each of whom, at all relevant times for the purposes of the Tax Act,
| • | is not resident in Canada, |
| • | does not use or hold, and is not deemed to use or hold, Common Shares in connection with carrying on a business in Canada, |
| • | is not an “authorized foreign bank,” and |
| • | is not an insurer that carries on business in Canada and elsewhere, |
(each a “Non-resident Holder”).
Disposition of Common Shares
A Non-resident Holder who disposes or is deemed to dispose of a Common Share generally will not be subject to tax under the Tax Act in respect of any capital gain, or entitled to deduct any capital loss, thereby realized unless the Common Share, at the time of the disposition,
| • | is “taxable Canadian property,” and |
| • | is not “treaty-protected property,” |
of the Non-resident Holder.
Generally, a Non-resident Holder’s Common Share should not be taxable Canadian property to the Non-resident Holder at the time of disposition if at that time the Common Shares are listed on a designated stock exchange (which currently includes the TSX and the Nasdaq) unless, at the time of disposition or at any time in the preceding 60 months,
| • | the Non-resident Holder, one or more persons with whom the Non-resident Holder did not deal at arm’s length for the purposes of the Tax Act, or one or more partnerships in which the Non-resident Holder or persons with whom the Non-resident Holder did not deal at arm’s length holds or held a membership interest (either directly or indirectly through one or more partnerships), alone or in any combination owned 25% or more of the issued shares of any class of shares of Aurinia, and |
| • | the Common Share derived more than 50% of its fair market value directly or indirectly from one, or any combination of, real or immovable property situated in Canada, “Canadian resource properties,” “timber resource properties,” or options in respect of, interests in, or for civil law purposes rights in, any such property, whether or not the property exists. |
Generally, a Non-resident Holder’s Common Shares will be treaty-protected property at the time of disposition if, at that time, the terms of a tax treaty between Canada and another country exempt the Non-resident Holder from tax under Part I of the Tax Act on any gain from the disposition of the Common Shares.
Non-resident Holders should consult their own tax advisers regarding whether their Common Shares are taxable Canadian property or treaty-protected property.
A Non-resident Holder who disposes or is deemed to dispose of a Common Share in a taxation year at a time when the Common Share is taxable Canadian property and is not treaty–protected property of the Non-resident Holder generally will be required to file a Canadian tax return to report the disposition. The Non-resident Holder generally will be required to include any resulting taxable capital gain in the Non-resident Holder’s taxable income earned in Canada for the taxation year, and entitled to deduct any resulting allowable capital loss from
S-22
taxable capital gains included in the Non-resident Holder’s taxable income earned in Canada for the year or, to the extent not so deductible, in any of the Non-resident Holder’s three preceding taxation years or any subsequent taxation year, subject to the detailed rules regarding the deductibility of allowable capital losses in the Tax Act.
Dividends
A Non-resident Holder to whom a dividend is or is deemed to be paid or credited on the Non-resident Holder’s Common Shares will generally be subject to Canadian withholding tax equal to 25% of the gross amount of the dividend, or such lower rate as may be provided by an applicable income tax treaty between Canada and another country. The rate of withholding tax under the Canada-U.S. Income Tax Convention (1980) (the “U.S. Treaty”) applicable to a dividend paid or credited to a Non-resident Holder who beneficially owns the dividend, and is a resident of the United States under the U.S. Treaty and entitled to its benefits, is 5% if the Non-resident Holder is a company that owns (or is considered to own) at least 10% of Aurinia’s voting stock, and 15% in any other case.
We are registering the Registrable Common Shares to permit the resale of the Registrable Common Shares by the Warrantholders, from time to time, after the date of this prospectus supplement in the United States. We will not receive any of the proceeds from the sale by the Warrantholder of the Registrable Common Shares.
The Warrantholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling Registrable Common Shares or interests in Registrable Common Shares received after the date of this prospectus supplement from a Warrantholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their Registrable Common Shares or interests in Registrable Common Shares on any stock exchange, market or trading facility on which the Registrable Common Shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The Warrantholders may use any one or more of the following methods when disposing of Registrable Common Shares or interests therein:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the Registrable Common Shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | short sales effected after the date the registration statement of which this prospectus is a part is declared effective by the SEC; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| • | broker-dealers may agree with the Warrantholders to sell a specified number of such Registrable Common Shares at a stipulated price per Registrable Common Share; |
| • | a combination of any such methods of sale; and |
| • | any other method permitted by applicable law. |
S-23
The Warrantholders may, from time to time, pledge or grant a security interest in some or all of the Registrable Common Shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the Registrable Common Shares, from time to time, under this prospectus supplement, or under an amendment to this prospectus supplement under General Instruction II.L. of Form F-10. The Warrantholders also may transfer the Registrable Common Shares in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus supplement.
In connection with the sale of the Registrable Common Shares or interests therein, the Warrantholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the Registrable Common Shares in the course of hedging the positions they assume. The Warrantholders may also sell Registrable Common Shares short and deliver these securities to close out their short positions, or loan or pledge the Registrable Common Shares to broker-dealers that in turn may sell these securities. The Warrantholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of Registrable Common Shares offered by this prospectus supplement, which Registrable Common Shares such broker-dealer or other financial institution may resell pursuant to this prospectus supplement.
The aggregate proceeds to the Warrantholders from the sale of the Registrable Common Shares offered by them will be the purchase price of the Registrable Common Shares less discounts or commissions, if any. Each of the Warrantholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of Registrable Common Shares to be made directly or through agents. We will not receive any of the proceeds from this Offering. Upon any exercise of the June 2016 Warrants or December 2016 Warrants by payment of cash, however, we will receive the exercise price of the warrants.
We have agreed under the Registration Rights Agreement to pay all fees and expenses incident to the registration of the Registrable Common Shares, other than the fees and disbursements of counsel to the applicable Warrantholders.
The Warrantholders also may resell all or a portion of the Registrable Common Shares in open market transactions in reliance upon Rule 144 or Rule 904 of Regulation S under the U.S. Securities Act, provided that they meet the criteria and conform to the requirements of that rule.
The Warrantholders and any underwriters, broker-dealers or agents that participate in the sale of the Registrable Common Shares or interests therein may be “underwriters” within the meaning of Section 2(a)(11) of the U.S. Securities Act. Any discounts, commissions, concessions or profit they earn on any resale of the Common Shares may be underwriting discounts or commissions under the U.S. Securities Act. Warrantholders who are “underwriters” within the meaning of Section 2(a)(11) of the U.S. Securities Act will be subject to the prospectus delivery requirements of the U.S. Securities Act. To the extent required, the Registrable Common Shares to be sold, the names of the Warrantholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an amendment to this prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus supplement.
In order to comply with the securities laws of some states, if applicable, the Registrable Common Shares may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the Registrable Common Shares may not be sold unless they have been registered or qualified for sale or an exemption from registration or qualification is available and is complied with.
This prospectus supplement has not been filed in respect of, and will not qualify, any distribution of the Registrable Common Shares in British Columbia or in any other province or territory of Canada at any time.
S-24
We have advised the Warrantholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of Registrable Common Shares in the market and to the activities of the Warrantholders and their affiliates. In addition, to the extent applicable we will make copies of this prospectus supplement (as it may be amended from time to time) available to the Warrantholders for the purpose of satisfying the prospectus delivery requirements of the U.S. Securities Act. The Warrantholders may indemnify any broker-dealer that participates in transactions involving the sale of the Registrable Common Shares against certain liabilities, including liabilities arising under the U.S. Securities Act.
We have agreed to indemnify the Warrantholders that are party to the Registration Rights Agreement against liabilities, including liabilities under the U.S. Securities Act and state securities laws, relating to the registration of the Registrable Common Shares offered by this prospectus supplement.
There can be no assurance that any Warrantholder will sell any or all of the Registrable Common Shares registered pursuant to the registration statement, of which this prospectus supplement and the accompanying prospectus form a part.
Hyuek Joon Lee, David Jayne, George Milne, Lorin Jeffry Randall and Joseph Hagan, each a member of our board of directors, reside outside of Canada and have appointed an agent for service of process in Canada, as set out in the table below.
| Name of Person |
Name and Address of Agent | |
| Hyuek Joon Lee, David Jayne, George Milne, Lorin Jeffry Randall and Joseph Hagan | Borden Ladner Gervais LLP, 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia, Canada V7X 1T2 |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-10 under the U.S. Securities Act of 1933, as amended, relating to the offering of our securities, of which this prospectus supplement forms a part. This prospectus supplement does not contain all of the information set forth in the registration statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC. Reference is made to such registration statement and the exhibits thereto for further information with respect to us and the Common Shares.
We are required to file with the various securities commissions or similar authorities in each of the applicable provinces and territories of Canada, annual and quarterly reports, material change reports and other information. We are also an SEC registrant subject to the informational requirements of the U.S. Securities Exchange Act of 1934, as amended, and, accordingly, file with, or furnish to, the SEC certain reports and other information. Under the multi-jurisdictional disclosure system adopted by the United States and Canada, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ from those in the United States. You may read and copy any document we file with or furnish to the SEC at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the same documents from the public reference room by paying a fee. Please call the SEC at 1-800-SEC-0330 or contact them at www.sec.gov for further information on the public reference room and copying charges.
S-25
ENFORCEABILITY OF CERTAIN CIVIL LIABILITIES
We are a corporation incorporated and existing under the laws of the Province of Alberta. Most of our officers and directors and some of the experts named in this prospectus supplement are not residents of the United States, and many of our assets and all or a substantial portion of assets of such persons are located outside of the United States. We have appointed CT Corporation System as our agent for service of process in the United States. We have also filed with the SEC, concurrently with the registration statement on Form F-10 relating to the accompanying prospectus, an appointment of agent for service of process on Form F-X. Under the Form F-X, we appointed CT Corporation System as our agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC and any civil suit or action brought against or involving us in a United States court arising out of or related to or concerning the Offering.
However, it may be difficult for United States investors to effect service of process within the United States upon those officers or directors who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon Aurinia’s civil liability and the civil liability of such officers or directors under United States federal securities laws or the securities or “blue sky” laws of any state within the United States. We have been advised by our Canadian counsel, Borden Ladner Gervais LLP, that, subject to certain limitations, a judgment of a United States court predicated solely upon civil liability under United States federal securities laws may be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. Aurinia has also been advised by Borden Ladner Gervais LLP, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of liability predicated solely upon United States federal securities laws.
Certain legal matters in connection with the Offering will be passed upon on our behalf by Borden Ladner Gervais LLP with respect to Canadian legal matters and by Cooley LLP, Palo Alto, California with respect to U.S. legal matters.
The partners and associates of Borden Ladner Gervais LLP (Canadian counsel for Aurinia), as a group, beneficially own, directly or indirectly, less than one percent of the outstanding securities of Aurinia.
PricewaterhouseCoopers LLP, our auditor, issued an auditor’s report dated March 13, 2018 in respect of our consolidated financial statements, which comprise the consolidated statements of financial position as at December 31, 2017 and December 31, 2016, and the consolidated statements of operations and comprehensive loss, consolidated statements of changes in shareholders’ equity (deficit) and cash flows for the years ended December 31, 2017 and December 31, 2016, and the related notes. PricewaterhouseCoopers LLP has advised us that they are independent with respect to Aurinia Pharmaceuticals Inc. within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of Alberta and the rules of the U.S. Securities and Exchange Commission.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment, irrespective of the determination at a later date of the
S-26
purchase price of the securities distributed. In several of the provinces, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, damages if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal adviser. Rights and remedies may also be available to purchasers under U.S. law; purchasers may wish to consult with a U.S. lawyer for particulars of these rights.
S-27
CERTIFICATE OF AURINIA PHARMACEUTICALS INC.
Dated: April 5, 2018
The short form prospectus, together with the documents incorporated in the prospectus by reference, as supplemented by the foregoing, constitutes full, true and plain disclosure of all material facts relating to the securities offered by the prospectus and this supplement as required by the securities legislation of British Columbia, Alberta and Ontario.
| (signed) Richard Glickman |
(signed) Dennis Bourgeault | |
| Richard Glickman | Dennis Bourgeault | |
| President and Chief Executive Officer | Chief Financial Officer |
On Behalf of the Board of Directors
| (signed) Lorin Jeffrey Randall |
(signed) Benjamin Rovinski | |
| Lorin Jeffrey Randall | Benjamin Rovinski | |
| Director | Director |
C-1
This short form prospectus has been filed under legislation in British Columbia, Alberta and Ontario, that permits certain information about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully for sale and therein only by persons permitted to sell such securities.
Information has been incorporated by reference in this short form prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of Aurinia Pharmaceuticals Inc. at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, Canada, Telephone: (604) 632-3473 and are also available electronically at www.sedar.com and www.sec.gov.
SHORT FORM BASE SHELF PROSPECTUS
| New Issue and Secondary Offering | March 26, 2018 |

AURINIA PHARMACEUTICALS INC.
US $250,000,000
Common Shares
Warrants
Subscription Receipts
Debt Securities
Units
This short form base shelf prospectus (the “Prospectus”) relates to the issue and sale from time to time, during the 25-month period that this Prospectus, including any amendments hereto, remains effective, of (i) common shares (“Common Shares”) in the capital of Aurinia Pharmaceuticals Inc. (“we”, “us”, “our”, or “Aurinia”) (ii) warrants to purchase Common Shares (“Warrants”), (iii) subscription receipts that entitle the holder to receive upon satisfaction of certain release conditions, and for no additional consideration, Common Shares or Warrants (“Subscription Receipts”), (iv) debt securities (“Debt Securities”); (v) securities comprised of more than one of Common Shares, Warrants, Subscription Receipts and/or Debt Securities, offered together as a unit (“Units”) (collectively, the “Securities”) or any combination of such Securities in one or more series or issuances, with a total offering price of such Securities, in the aggregate, of up to US$250,000,000 (or its equivalent in Canadian dollars or any other currency). The Securities may either be offered by us or by our securityholders. The Securities may be offered separately or together, in amounts, at prices and on terms to be determined based on market conditions at the time of the sale and set out in an accompanying prospectus supplement.
The Common Shares are listed on the Nasdaq Stock Market, or Nasdaq, under the symbol “AUPH” and on the Toronto Stock Exchange, or TSX, under the symbol “AUP”. On March 23, 2018, the last trading day prior to the filing of this Prospectus, the closing price of the Common Shares was US$5.25 on Nasdaq and CDN$6.76 on the TSX.
All information permitted under securities legislation to be omitted from this Prospectus will be contained in one or more prospectus supplements (each, a “Prospectus Supplement”) that will be delivered to purchasers together with this Prospectus. Each Prospectus Supplement will be incorporated by reference into this Prospectus for the purposes of securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains. You should read this Prospectus and any applicable Prospectus Supplement carefully before you invest in any Securities issued pursuant to this Prospectus.
Unless otherwise specified in an applicable Prospectus Supplement, Warrants, Subscription Receipts, Debt Securities and Units (other than Common Shares included in the Units) will not be listed on any securities or stock exchanges or on any automated dealer quotation system. There is currently no market through which our Securities, other than the Common Shares, may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of our Securities, other than the Common Shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these Securities and the extent of issuer regulation. See “Risk Factors”.
The Securities may be sold pursuant to this Prospectus through underwriters, dealers or agents that we designate from time to time or directly by us at amounts and prices and other terms that we determine. In connection with any underwritten offering of the Securities, the underwriters may over-allot or effect transactions which stabilize or maintain the market price of the Securities offered. Such transactions, if commenced, may discontinue at any time. See “Plan of Distribution”. A Prospectus Supplement relating to a particular offering of Securities will set out the names of any underwriters, dealers or agents involved in the sale of the Securities, the amount of Securities, if any, to be purchased by underwriters, the plan of distribution for such Securities, including the net proceeds we expect to receive from the sale of such Securities, if any, the amounts and prices at which such Securities are to be sold and the compensation of such underwriters, dealers of agents.
Our registered office is located at #201, 17904 – 105 Avenue, Edmonton, Alberta T5S 2H5, Canada. Our head office is located at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8, Canada.
Agent for Service of Process
Hyuek Joon Lee, David Jayne, George Milne, Lorin Jeffry Randall and Joseph Hagan are our directors that reside outside of Canada. Each of these directors has appointed the following agent for service of process in Canada:
| Name of Person |
Name and Address of Agent | |
| Hyuek Joon Lee, David Jayne, George Milne, Lorin Jeffry Randall and Joseph Hagan | Borden Ladner Gervais LLP 1200 Waterfront Centre 200 Burrard Street, P.O. Box 48600 Vancouver, BC V7X 1T2 Attention : Stephen P. Robertson |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
Investing in the Securities involves a high degree of risk. You should carefully read the “Risk Factors” section beginning on page 13 of this Prospectus.
This offering is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system adopted by the United States, to prepare this Prospectus in accordance with the disclosure requirements of Canada. Prospective investors should be aware that such requirements are different from those of the United States. Financial statements included or incorporated herein, if any, have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and may be subject to Canadian auditing and auditor independence standards, and thus may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the acquisition of the securities described herein may have tax consequences both in the United States and in Canada. Such consequences are not described herein and may not be fully described in any applicable Prospectus Supplement. You should read the tax discussion in any Prospectus Supplement with respect to a particular offering and consult your own tax advisor with respect to your own particular circumstances.
The enforcement by investors of civil liabilities under U.S. federal securities laws may be affected adversely by the fact that we are incorporated under the provincial laws of Alberta, most of the our officers and directors and the experts named in this Prospectus are Canadian residents or residents outside of the United States, and all or a substantial portion of our assets and the assets of our officers, directors and experts are located outside the United States.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION NOR HAS THE COMMISSION PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
No underwriter has been involved in the preparation of this Prospectus or performed any review of the contents of this Prospectus.
ii
| 1 | ||||
| 1 | ||||
| 2 | ||||
| 6 | ||||
| 8 | ||||
| 8 | ||||
| 8 | ||||
| 9 | ||||
| 13 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
| 29 | ||||
| 30 | ||||
| 30 | ||||
| 32 | ||||
| 35 | ||||
| 37 | ||||
| 37 | ||||
| 38 | ||||
| 41 | ||||
| 41 | ||||
| 41 | ||||
| 41 | ||||
| 42 | ||||
| 42 | ||||
| 42 | ||||
| 43 | ||||
| 44 | ||||
You should rely only on the information contained or incorporated by reference in this Prospectus and any applicable Prospectus Supplement and on the other information included in the registration statement of which this Prospectus forms a part. We have not authorized anyone to provide you with different or additional information. If anyone provides you with different or additional information, you should not rely on it. We are not making an offer to sell or seeking an offer to buy the Securities offered pursuant to this Prospectus in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this Prospectus or any applicable Prospectus Supplement is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this Prospectus or any applicable Prospectus Supplement or of any sale of Securities pursuant thereto. Our business, financial condition, results of operations and prospects may have changed since those dates.
Market data and certain industry forecasts used in this Prospectus or any applicable Prospectus Supplement and the documents incorporated by reference in this Prospectus or any applicable Prospectus Supplement were obtained from market research, publicly available information and industry publications. We believe that these sources are generally reliable, but the accuracy and completeness of this information is not guaranteed. We have not independently verified such information, and we do not make any representation as to the accuracy of such information.
In this Prospectus and any Prospectus Supplement, unless otherwise indicated, all dollar amounts and references to “US$” are to U.S. dollars and references to “CDN$” are to Canadian dollars. This Prospectus and the documents incorporated by reference contain translations of some Canadian dollar amounts into U.S. dollars solely for your convenience. See “Exchange Rate Information”.
In this Prospectus and in any Prospectus Supplement, unless the context otherwise requires, references to “we”, “us”, “our”, or “Aurinia”, refer to Aurinia Pharmaceuticals Inc., either alone or together with its subsidiaries.
PRESENTATION OF FINANCIAL INFORMATION
Our consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”).
A statement is forward-looking when it uses what we know and expect today to make a statement about the future. Forward-looking statements may include words such as “anticipate”, “believe”, “intend”, “expect”, “goal”, “may”, “outlook”, “plan”, “seek”, “project”, “should”, “strive”, “target”, “could”, “continue”, “potential” and “estimated”, or the negative of such terms or comparable terminology. You should not place undue reliance on the forward-looking statements, particularly those concerning anticipated events relating to the development, clinical trials, regulatory approval, and marketing of our product and the timing or magnitude of those events, as they are inherently risky and uncertain.
Securities laws encourage companies to disclose forward-looking information so that investors can get a better understanding our future prospects and make informed investment decisions. These statements, made either in this Prospectus or a document incorporated by reference in this Prospectus, may include, without limitation:
| • | our belief that the Phase IIb lupus nephritis AURA- LV (“AURA”) clinical trial had positive results; |
| • | our belief that we have sufficient cash resources to adequately fund operations through Phase III lupus nephritis (“AURORA”) clinical trial results and regulatory submission; |
| • | our belief that confirmatory data generated from the single AURORA clinical trial and the recently completed AURA clinical trial should support regulatory submissions in the United States, Europe and Japan and the timing of such including the New Drug Applications submission in the United States; |
| • | our belief that recently granted formulation patents regarding the delivery of voclosporin to the ocular surface for conditions such as dry eye have the potential to be of therapeutic value; |
| • | our plans and expectations and the timing of commencement, enrollment, completion and release of results of clinical trials; |
| • | our current forecast for the cost of the AURORA clinical trial and the continuation study; |
| • | our intention to seek regulatory approvals in the United States, Europe and Japan for voclosporin and anticipated timing of receiving approval; |
| • | our intention to demonstrate that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class status for the treatment of lupus nephritis (“LN”) outside of Japan; |
| • | our belief in voclosporin being potentially a best-in-class CNI (as defined below) with robust intellectual property exclusivity and the benefits over existing commercially available CNIs; |
| • | our belief that voclosporin has further potential to be effectively used across a range of therapeutic autoimmune areas including focal segmental glomerularsclerosis (“FSGS”) and dry eye syndrome (“DES”); |
| • | our intention to initiate a Phase II clinical trial for voclosporin in FSGS patients and the timing for commencement and for data availability for the same; |
| • | our intention to commence a Phase IIa tolerability study of voclosporin ophthalmic solution (“VOS”) and the timing for commencement and for data availability for the same; |
| • | statements concerning the anticipated commercial potential of voclosporin for the treatment of LN, FSGS and DES; |
| • | our belief that the expansion of the renal franchise could create significant value for shareholders; |
| • | our intention to use the net proceeds from financings for various purposes; |
| • | our belief that Aurinia’s current financial resources are sufficient to fund all existing programs, the new indication expansion and new product development work and supporting operations into 2020. |
2
| • | our plans to generate future revenues from products licensed to pharmaceutical and biotechnology companies |
| • | statements concerning partnership activities and health regulatory discussions; |
| • | statements concerning the potential market for voclosporin; |
| • | our ability to take advantage of financing opportunities if and when needed; |
| • | our belief that VOS has the potential to compete in he multi-billion-dollar human prescription dry eye market; |
| • | our intention to seek additional corporate alliances and collaborative agreements to support the commercialization and development of our product; and |
| • | our belief that the annualized pricing for voclosporin could range between $45,000-$100,000 in the United States. |
Such statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based on a number of estimates and assumptions that, while considered reasonable by management, as at the date of such statements, are inherently subject to significant business, economic, competitive, political, regulatory, legal, scientific and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The factors and assumptions used by management to develop such forward-looking statements include, but are not limited to:
| • | the assumption that we will be able to obtain approval from regulatory agencies on executable development programs with parameters that are satisfactory to us; |
| • | the assumption that recruitment to clinical trials will occur as projected; |
| • | the assumption that we will successfully complete our clinical programs on a timely basis, including conducting the required AURORA clinical trial and meet regulatory requirements for approval of marketing authorization applications and new drug approvals, as well as favourable product labelling; |
| • | the assumption that the planned studies will achieve positive results; |
| • | the assumptions regarding the costs and expenses associated with Aurinia’s clinical trials; |
| • | the assumption the regulatory requirements and commitments will be maintained; |
| • | the assumption that we will be able to meet GMP standards and manufacture and secure a sufficient supply of voclosporin on a timely basis to successfully complete the development and commercialization of voclosporin; |
| • | the assumptions on the market value for the LN program; |
| • | the assumption that our patent portfolio is sufficient and valid; |
| • | the assumption that we will be able to extend our patents on terms acceptable to us; |
| • | the assumptions on the market |
| • | the assumption that there is a potential commercial value for other indications for voclosporin; |
| • | the assumption that market data and reports reviewed by us are accurate; |
| • | the assumption that another company will not create a substantial competitive product for Aurinia’s LN business without violating Aurinia’s intellectual property rights; |
| • | the assumptions on the burn rate of Aurinia’s cash for operations; |
| • | the assumption that our current good relationships with our suppliers, service providers and other third parties will be maintained; |
3
| • | the assumption that we will be able to attract and retain a sufficient amount of skilled staff and/or |
| • | the assumptions relating to the capital required to fund operations through AURORA clinical trial results and regulatory submission. |
It is important to know that:
| • | actual results could be materially different from what we expect if known or unknown risks affect our business, or if our estimates or assumptions turn out to be inaccurate. As a result, we cannot guarantee that any forward-looking statement will materialize and, accordingly, you are cautioned not to place undue reliance on these forward-looking statements. |
| • | forward-looking statements do not take into account the effect that transactions or non-recurring or other special items announced or occurring after the statements are made may have on our business. For example, they do not include the effect of mergers, acquisitions, other business combinations or transactions, dispositions, sales of assets, asset write-downs or other charges announced or occurring after the forward-looking statements are made. The financial impact of such transactions and non-recurring and other special items can be complex and necessarily depends on the facts particular to each of them. Accordingly, the expected impact cannot be meaningfully described in the abstract or presented in the same manner as known risks affecting our business. |
The factors discussed below and other considerations discussed in the “Risk Factors” section of this Prospectus could cause our actual results to differ significantly from those contained in any forward-looking statements.
Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to differ materially from any assumptions, further results, performance or achievements expressed or implied by such forward-looking statements. Important factors that could cause such differences include, among other things, the following:
| • | the need for additional capital in the longer term to fund our development programs and the effect of capital market conditions and other factors on capital availability; |
| • | competition in our market place; |
| • | difficulties, delays, or failures we may experience in the conduct of and reporting of results of our clinical trials for voclosporin; |
| • | difficulties in meeting Good Manufacturing Practice (“GMP”) standards and the manufacturing and securing a sufficient supply of voclosporin on a timely basis to successfully complete the development and commercialization of voclosporin; |
| • | difficulties, delays or failures in obtaining regulatory approvals for the initiation of clinical trials; |
| • | difficulties in gaining alignment among the key regulatory jurisdictions, European Medicines Agency (“EMA”), Food and Drug Administration (“FDA”) and Pharmaceutical and Medical Devices Agency (“PMDA”), which may require further clinical activities; |
| • | difficulties, delays or failures in obtaining regulatory approvals to market voclosporin; |
| • | not being able to extend our patent portfolio for voclosporin; |
| • | difficulties we may experience in completing the development and commercialization of voclosporin; |
| • | the market for the LN business may not be as we have estimated; |
| • | insufficient acceptance of and demand for voclosporin; |
| • | difficulties obtaining adequate reimbursements from third party payors; |
| • | difficulties obtaining formulary acceptance; |
4
| • | competitors may arise with similar products; |
| • | product liability, patent infringement and other civil litigation; |
| • | injunction, court orders, regulatory and other enforcement actions; |
| • | we may have to pay unanticipated expenses, and/or estimated costs for clinical trials or operations may be underestimated, resulting in our having to make additional expenditures to achieve our current goals; |
| • | difficulties, restrictions, delays, or failures in obtaining appropriate reimbursement from payers for voclosporin; and/or |
| • | difficulties we may experience in identifying and successfully securing appropriate vendors to support the development and commercialization of our product. |
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These forward-looking statements are made as of the date of this Prospectus or, in the case of documents incorporated by reference in this Prospectus, as of the date of such documents or, in the case of any Prospectus Supplement, as of the date of such Prospectus Supplement and we disclaim any intention and have no obligation or responsibility, except as required by law, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
5
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada which have also been filed with, or furnished to, the United States Securities and Exchange Commission (the “SEC”). Copies of the documents incorporated by reference in this Prospectus and not delivered with this Prospectus may be obtained on request without charge from our Corporate Secretary at 1200 Waterfront Centre, 200 Burrard Street, P.O. Box 48600, Vancouver, British Columbia V7X 1T2, Canada, Telephone: (604) 632-3473 or by accessing the disclosure documents through the Internet on the Canadian System for Electronic Analysis and Retrieval, or SEDAR, at www.sedar.com. Documents filed with, or furnished to, the SEC are available through the SEC’s Electronic Data Gathering and Retrieval System, or EDGAR, at www.sec.gov.
The following documents, filed with the securities commissions or similar regulatory authorities in British Columbia, Alberta and Ontario, and filed with, or furnished to, the SEC are specifically incorporated by reference into, and form an integral part of, this Prospectus:
| (a) | our annual information form dated March 13, 2018 for the fiscal year ended December 31, 2017; |
| (b) | our audited consolidated financial balance sheets as at December 31, 2017 and 2016 and the consolidated statements of operations and comprehensive loss, changes in shareholders’ equity and cash flows for the two-year period ended December 31, 2017, including notes thereto and the auditors’ report thereon, as filed on March 15, 2018; |
| (c) | our management’s discussion and analysis of financial condition and results of operations for the year ended December 31, 2017; and |
| (d) | our management information circular dated May 12, 2017 in connection with the annual general meeting of our shareholders on June 21, 2017. |
Any documents of the type described in Section 11.1 of Form 44-101F1 Short Form Prospectuses that we have filed with a securities commission or similar authority in any jurisdiction of Canada subsequent to the date of this Prospectus and prior to the expiry of this Prospectus, or the completion of the issuance of Securities pursuant hereto, will be deemed to be incorporated by reference into this Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus is filed with, or furnished to, the SEC pursuant to the Securities Exchange Act of 1934, as amended (the “Exchange Act”), after the date of this Prospectus, that document or information will be deemed to be incorporated by reference as an exhibit to the registration statement of which this Prospectus forms a part (in the case of a report on Form 6-K, if and to the extent expressly provided therein).
A Prospectus Supplement containing the specific terms of any offering of the Securities will be delivered to purchasers of such Securities together with this Prospectus and will be deemed to be incorporated by reference in this Prospectus as of the date of that Prospectus Supplement and only for the purposes of the offering of the Securities to which that Prospectus Supplement pertains.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded, for the purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to
6
make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this Prospectus.
Upon filing a new annual information form and the related annual financial statements and management’s discussion and analysis with applicable securities regulatory authorities during the currency of this Prospectus, the previous annual information form, the previous annual financial statements and management’s discussion and analysis and all quarterly financial statements, supplemental information and material change reports filed prior to the commencement of our financial year in which the new annual information form is filed will be deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of the Securities under this Prospectus. Upon interim consolidated financial statements and the accompanying management’s discussion and analysis being filed by us with the applicable securities regulatory authorities during the duration of this Prospectus, all interim consolidated financial statements and the accompanying management’s discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of Securities under this Prospectus. Upon a new management information circular in connection with an annual meeting of shareholders being filed by us with the applicable securities regulatory authorities during the duration of this Prospectus, the management information circular filed in connection with the previous annual meeting of shareholders will be deemed no longer to be incorporated by reference in this Prospectus for purposes of future offers and sales of Securities under this Prospectus.
7
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been or will be filed with the SEC as part of the registration statement of which this Prospectus forms a part: (i) the documents listed under the heading “Documents Incorporated by Reference”; (ii) powers of attorney from our directors and officers; and (iii) the consent of PricewaterhouseCoopers LLP.
The following table sets forth for each period indicated: (i) the exchange rates in effect at the end of the period; (ii) the high and low exchange rates during such period; and (iii) the average exchange rates for such period, for one Canadian dollar, expressed in U.S. dollars, as quoted by the Bank of Canada.
| Year Ended December 31 | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| US$ | US$ | US$ | ||||||||||
| Closing |
0.7971 | 0.7448 | 0.7225 | |||||||||
| High |
0.8245 | 0.7972 | 0.8527 | |||||||||
| Low |
0.7276 | 0.6854 | 0.7148 | |||||||||
| Average |
0.7708 | 0.7548 | 0.7820 | |||||||||
On March 23, 2018, the closing exchange rate as quoted by the Bank of Canada was CDN$1.00 = US$0.778.
We are a clinical stage biopharmaceutical company with a head office at #1203-4464 Markham Street, Victoria, British Columbia V8Z 7X8. Our registered office is located at #201, 17904-105 Avenue, Edmonton, Alberta T5S 2H5 where the finance function is performed.
We are organized under the Business Corporations Act (Alberta). The Common Shares are currently listed and traded on the Nasdaq Global Market (“Nasdaq”) under the symbol “AUPH” and on the Toronto Stock Exchange (“TSX”) under the symbol “AUP”. Our primary business is the development of a therapeutic drug to treat autoimmune diseases, in particular LN.
We have the following wholly-owned subsidiaries: Aurinia Pharma U.S., Inc. (Delaware incorporated) and Aurinia Pharma Limited (UK incorporated).
Our By-Law No. 2 was amended at a shareholder’s meeting held on August 15, 2013 to include provisions requiring advance notice for any nominations of directors by shareholders, which are described further in our most recent information circular.
8
SUMMARY DESCRIPTION OF BUSINESS
We are focused on the development of our novel therapeutic immunomodulating drug candidate, voclosporin, for the treatment of LN, FSGS and DES. Voclosporin is a next generation CNI which has clinical data in over 2,400 patients across multiple indications. It has been also previously studied in kidney rejection following transplantation, psoriasis and in various forms of uveitis (an ophthalmic disease).
Legacy CNIs have demonstrated efficacy for a number of conditions, including LN, transplant, DES and other autoimmune diseases; however, side effects exist which can limit their long-term use and tolerability. Some clinical complications of legacy CNIs include hypertension, hyperlipidemia, diabetes, and both acute and chronic nephrotoxicity.
Voclosporin is an immunosuppressant, with a synergistic and dual mechanism of action that has the potential to improve near- and long-term outcomes in LN when added to mycophenolate mofetil (“MMF”), although not approved for such, the current standard of care for LN. By inhibiting calcineurin, voclosporin reduces cytokine activation and blocks interleukin IL-2 expression and T-cell mediated immune responses. Voclosporin also potentially stabilizes disease modifying podocytes, which protects against proteinuria. Voclosporin is made by a modification of a single amino acid of the cyclosporine molecule which has shown a more predictable pharmacokinetic and pharmacodynamic relationship, an increase in potency, an altered metabolic profile, and easier dosing without the need for therapeutic drug monitoring. Clinical doses of voclosporin studied to date range from 13 – 70 mg BID. The mechanism of action of voclosporin, a CNI, has been validated with certain first generation CNIs for the prevention of rejection in patients undergoing solid organ transplants and in several autoimmune indications, including dermatitis, keratoconjunctivitis sicca, psoriasis, rheumatoid arthritis, and for LN in Japan. We believe that voclosporin possesses pharmacologic properties with the potential to demonstrate best-in-class differentiation with first-in-class regulatory approval status for the treatment of LN outside of Japan.
Based on published data, we believe the key potential benefits of voclosporin in the treatment of LN are as follows:
| • | increased potency compared to cyclosporine A, allowing lower dosing requirements and fewer off target effects; |
| • | limited inter and intra patient variability, allowing for easier dosing without the need for therapeutic drug monitoring; |
| • | less cholesterolemia and triglyceridemia than cyclosporine A; and |
| • | limited incidence of glucose intolerance and diabetes at therapeutic doses compared to tacrolimus. |
Lupus Nephritis
LN is an inflammation of the kidney caused by systemic lupus erythematosus (“SLE”) and represents a serious manifestation of SLE. SLE is a chronic, complex and often disabling disorder. SLE is highly heterogeneous, affecting a wide range of organs and tissue systems. Unlike SLE, LN has straightforward disease measures (readily assessable and easily identified by specialty treaters) where an early response correlates with long-term outcomes, measured by proteinuria. In patients with LN, renal damage results in proteinuria and/or hematuria and a decrease in renal function as evidenced by reduced estimated glomerular filtration rate (“eGFR”), and increased serum creatinine levels. eGFR is assessed through the Chronic Kidney Disease Epidemiology Collaboration equation. Rapid control and reduction of proteinuria in LN patients measured at 6 months shows a reduction in the need for dialysis at 10 years. LN can be debilitating and costly and if poorly controlled, can lead to permanent and irreversible tissue damage within the kidney. Recent literature suggests severe LN progresses to end-stage renal disease (“ESRD”), within 15 years of diagnosis in 10%-30% of patients, thus making LN a serious and potentially life-threatening condition. SLE patients with renal damage have a 14-fold increased risk of premature death, while SLE patients with ESRD have a greater than 60-fold increased risk of premature death.
9
Mean annual cost for patients (both direct and indirect) with SLE (with no nephritis) have been estimated to exceed $20,000 per patient, while the mean annual cost for patients (both direct and indirect) with LN who progress to intermittent ESRD have been estimated to exceed $60,000 per patient.
FSGS
FSGS is a lesion characterized by persistent scarring identified by biopsy and proteinuria. FSGS is a cause of Nephrotic Syndrome (“NS”) and is characterized by high morbidity. NS is a collection of symptoms that indicate kidney damage, including: large amounts of protein in the urine; low levels of albumin and higher than normal fat and cholesterol levels in the blood, and edema. Similar to LN, early clinical response and reduction of proteinuria is thought to be critical to long-term kidney health and outcomes.
FSGS is likely the most common primary glomerulopathy and the most common primary glomerulopathy leading to ESRD. The incidence of FSGS and ESRD due to FSGS are increasing as time goes on. Precise estimates of incidence and prevalence are difficult to determine. According to NephCure Kidney International, more than 5400 patients are diagnosed with FSGS every year; however, this is considered an underestimate because a limited number of biopsies are performed. The number of FSGS cases are rising more than any other cause of NS and the incidence of FSGS is increasing through disease awareness and improved diagnosis. FSGS occurs more frequently in adults than in children and is most prevalent in adults 45 years or older. FSGS is most common in people of African American and Asian descent. It has been shown that the control of proteinuria is important for long-term dialysis-free survival of these patients. Currently, there are no approved therapies for FSGS in the United States and Europe.
DES
DES, or keratoconjunctivitis sicca, is a chronic disease in which a lack of moisture and lubrication on the eye’s surface results in irritation and inflammation of the eye. DES is a multifactorial, heterogeneous disease estimated to affect greater than 20 million people in the United States.
LN Standard of Care
While at Aspreva, certain members of Aurinia’s management team executed the ALMS study which established CellCept® as the current standard of care for treating LN. The ALMS study was published in 2009 in the Journal of the American Society of Nephrology and in 2011 in the New England Journal of Medicine.
The American College of Rheumatology recommends that intravenous cyclophosphamide or MMF/CellCept® be used as first-line immunosuppressive therapy for LN. Despite their use, the ALMS study showed that the vast majority of patients failed to achieve CR, and almost half failed to have a renal response at 24 weeks for both of these therapeutics. Based upon the results of the ALMS study, we believe that a better solution is needed to improve renal response rates for LN.
Despite CellCept® being the current SoC for the treatment of LN, it remains far from adequate with fewer than 20% of patients on therapy actually achieving disease remission after six months of therapy. Data suggests that a LN patient who does not achieve rapid disease remission upon treatment is more likely to experience renal failure or require dialysis at 10 years (Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ; the Collaborative Study Group. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3:46-53.). Therefore, it is critically important to achieve disease remission as quickly and as effectively as possible.
Based on available data from the AURA clinical trial, we believe that voclosporin has the potential to address the critical need for LN by controlling active disease rapidly, lowering the overall steroid burden, impacting extra-renal disease and doing so with a convenient oral twice-daily treatment regimen.
10
Market Potential and Commercial Considerations
Our target launch date for voclosporin as treatment for LN is late 2020 or early 2021. The global immunology market which covers autoimmune conditions including SLE is set to rise from $57.7 billion in 2015 to $75.4 billion by 2022 according to GBI Research with the United States accounting for half of the market. The growth in the market is driven by continued increase in prevalence and incidence of autoimmune conditions in addition to new market entrants that are targeting better outcomes. There is significant unmet need for new therapies specifically in SLE and LN.
We have conducted market research including claims database reviews (where available) and physician based research. Our physician research included approximately 900 rheumatologists and nephrologists across the United States, Europe and Japan to better define the potential market size, estimated pricing and treatment paradigms in those jurisdictions. Using the U.S. MarketScan® database (with approximately ~180,000,000 insured lives in the United States) there were 445,000 SLE patients in the database (between January 2006 and June 2016) based on specific SLE diagnosis codes. The National Institute of Diabetes and Digestive and Kidney Diseases estimates that up to 50% of people with SLE are diagnosed with LN. The Lupus Foundation of America estimates that 60% of people with lupus may develop kidney problems. Using claims database research and physician research, we believe the diagnosed range of LN patients to be approximately 125,000 to 200,000 in the United States and 150,000 to 215,000 for Europe and Japan combined. In the United States, Europe and Japan, one in five LN patients are thought to be undiagnosed due to referring physicians being inefficient and inaccurate in diagnosing the condition according to our research.
Similar to other autoimmune disorders, LN is a flaring and remitting disease. The destructive disease cycle people with LN go through is depicted below. The disease cycles from being in remission to being in flare, achieving partial remission and being back in remission. Treatment objectives between LN and other autoimmune diseases are remarkably similar. In other autoimmune conditions such as Multiple Sclerosis, Crohn’s, Rheumatoid Arthritis and SLE physicians goals are to induce/maintain a remission of disease, decrease frequency of hospital or ambulatory care visits and limit long term disability. In LN specifically, physicians are trying to avoid further kidney damage, dialysis, renal transplantation, and death. According to a physician survey, the frequency of LN flares amongst treated patients was approximately every 14 months across the United States and Europe. The ability to get patients into remission quickly correlates with better long-term kidney outcomes as noted above.
The population of people with LN will be in different cycles of their disease at any one time. Physicians currently use existing LN standard of care including immunosuppressants and high dose steroids to treat people with LN throughout the disease cycles including induction and maintenance phases. By studying voclosporin on top of an existing standard of care we are not seeking to displace current accepted treatment patterns. We feel that being additive to an existing standard of care in addition to the product being administered orally versus via infusion or injection can support a more rapid market adoption if approved.
11
Current annualized pricing (based on wholesale acquisition costs published by AnalySource® Reprinted with permission by First Databank, Inc. All rights reserved. © (2018)) for the treatments of other more prevalent autoimmune conditions such as Multiple Sclerosis, Crohn’s, Rheumatoid Arthritis and SLE ranges from $45,000-$100,000 in the United States. Wholesale acquisition cost is the manufacturer’s published catalog or list price for a drug product to wholesalers. We have conducted pricing research that studied a similar pricing range with payers and physicians and believe that pricing in this range can be achievable for voclosporin in the United States. Pricing for other autoimmune conditions is lower in Europe and Japan than it is in the United States driven by the specific country’s pricing and reimbursement processes. We expect that will be the case for voclosporin. We believe the United States will provide the largest market opportunity for the Company followed by Europe and Japan.
Recent Developments
Appointment of new board members
On February 8, 2018 we announced the appointment of Joseph P. “Jay” Hagan. to our board of directors. Mr. Hagan is currently the President and Chief Executive Officer of Regulus Therapeutics, having previously held the positions of Chief Operating Officer, Principal Financial Officer and Principal Accounting Officer.
On February 22, 2018 we announced the appointment of Michael Hayden, CM OBC MB ChB PhD FRCP (C) FRSC to our board of directors. Dr. Hayden was most recently the President of Global R&D and Chief Scientific Officer at Teva. Dr. Hayden is the co-founder of three biotechnology companies, including Aspreva, and currently sits on several boards. Dr. Hayden is a celebrated researcher, having focused his research primarily on genetic diseases.
Cash at December 31, 2017
At December 31, 2017 we had cash, cash equivalents and short term investments (bonds) on hand of $173.5 million. We believe this cash position will provide funding to complete the AURORA Phase III clinical trial with estimated costs to finish of approximately $53 million and complete the regulatory New Drug Approval submission with the FDA in early 2020 based on current expected timelines. We also expect to conduct a two year continuation study with an estimated cost of approximately $17 million to be incurred for the period into 2020. In addition our cash position is expected to allow us to conduct our planned Phase II studies for voclosporin in FSGS and VOS for dry eye syndrome, and conduct the required drug-drug interaction study between voclosporin and MMF, while also funding our corporate, administration and business development activities and working capital needs into 2020.
12
Investing in Securities involves a high degree of risk. In addition to the other information included or incorporated by reference in this Prospectus or any applicable Prospectus Supplement, you should carefully consider the risks described below before purchasing Securities. If any of the following risks actually occur, our business, financial condition and results of operations could materially suffer. As a result, the trading price of Common Shares could decline, and you might lose all or part of your investment. The risks set out below are not the only risks we face; risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially and adversely affect our business, financial condition and results of operations. You should also refer to the other information set forth or incorporated by reference in this Prospectus or any applicable Prospectus Supplement, including our consolidated financial statements and related notes.
Risks Relating to Aurinia’s Business
Clinical Trial Progress and Results – Heavy Dependence on Voclosporin
We have invested a significant portion of our time and financial resources in the development of voclosporin. Voclosporin is currently our only product candidate. We anticipate that our ability to generate revenues and meet expectations will depend on the successful development and commercialization of voclosporin. The successful development and commercialization of voclosporin will depend on several factors, including the following:
| • | successful completion of clinical programs, and in particular, the underway AURORA Phase 3 LN clinical trial; |
| • | receipt of marketing approvals from the FDA and other regulatory authorities with a commercially viable label; |
| • | securing and maintaining sufficient expertise and resources to help in the continuing development and eventual commercialization of voclosporin for autoimmune indications and/or transplant; |
| • | maintaining suitable manufacturing and supply agreements to ensure commercial quantities of the product through validated processes; and |
| • | acceptance and adoption of the product by the medical community and third-party payors. |
It is possible that we may decide to discontinue the development of voclosporin at any time for commercial, scientific, or regulatory reasons. If voclosporin is developed, but not marketed, we will have invested significant resources and our future operating results and financial conditions would be significantly adversely affected. If we are not successful in commercializing voclosporin, or significantly delayed in doing so, our business will be materially harmed and we may need to curtail or cease operations.
We may not be able to obtain required regulatory approvals for our product candidate and there is no assurance of successful development
We have not completed the development of any therapeutic products and in particular, voclosporin, and therefore there can be no assurance that any product will be successfully developed. Voclosporin has not received regulatory approval for our commercial use and sale for any indication, in any jurisdiction. We cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. In general, significant research and development and clinical studies are required to demonstrate the safety and effectiveness of our product before submission of any regulatory applications. We may never obtain the required regulatory approvals for our product in any indication. Product candidates require significant additional research and development efforts, including clinical trials, prior to regulatory approval and potential commercialization, however, there can be no assurance that the results of all required clinical trials will demonstrate that these product candidates are safe and effective or, even if the results of all required clinical trials do demonstrate that these product candidates are safe
13
and effective, or even if the results of the clinical trials are considered successful by us, that the regulatory authorities will not require us to conduct additional clinical trials before they will consider approving product candidates for commercial use. The FDA and other regulators have substantial discretion in the approval process.
Approval or consent by regulatory authorities to commence a clinical trial does not indicate that the device, drug, or treatment being studied can or will be approved. Of the large number of drugs in development, only a small percentage result in the submission of an application to the FDA and even fewer are approved for commercialization. The process of obtaining required approvals (such as, but not limited to, the approval of the FDA, the EMA, PMDA and Health Canada) is complex, expensive, time intensive, entails significant uncertainty and there can be no assurance that future products will be successfully developed, proven safe and effective in clinical trials or receive applicable regulatory approvals. Potential investors should be aware of the risks, problems, delays, expenses and difficulties which may be encountered by us in view of the extensive regulatory environment which controls our business. The regulatory review process typically varies in time, may take years to complete and approval is not guaranteed. Any approval might also contain significant limitations which may affect our ability to successfully develop its product candidate. Also, any regulatory approval once obtained, may be withdrawn. If regulatory approval is obtained in one jurisdiction, that does not necessarily mean that we will receive regulatory approval in all jurisdictions in which we may seek approval, or any regulatory approval obtained may not be as broad as what was obtained in other jurisdictions. However, the failure to obtain approval for our product candidate in one or more jurisdictions may negatively impact our ability to obtain approval in a different jurisdiction. If our development efforts for our product candidate are not successful or regulatory approval is not obtained in a timely fashion, on acceptable terms or at all, it will have a material adverse effect on the business, financial condition, and results of operations.
The results of our completed preclinical studies and clinical trials may not be indicative of future clinical trial results. A commitment of substantial resources to conduct time-consuming research, preclinical studies, and clinical trials will be required if we are to complete the development of our product.
There can be no assurance that unacceptable toxicities or adverse side effects will not occur at any time in the course of preclinical studies or human clinical trials or, if any products are successfully developed and approved for marketing, during commercial use of our product. The appearance of any such unacceptable toxicities or adverse side effects could interrupt, limit, delay, or abort the development of our product or, if previously approved, necessitate its withdrawal from the market. Furthermore, there can be no assurance that disease resistance or other unforeseen factors will not limit the effectiveness of our product. Any products resulting from our programs are not expected to be successfully developed or made commercially available in the near term and may not be successfully developed or made commercially available at all. Should our product prove to have insufficient benefit and/or have an unsafe profile, its development will likely be discontinued.
Our future performance will be impacted by a number of important factors, including, in the short-term, our ability to continue to generate cash flow from financings, and in the longer term, our ability to generate royalty or other revenues from licensed technology and bring new products to the market. Our future success will require efficacy and safety of our product and regulatory approval for the product. Future success of commercialization of any product is also dependent on our ability to obtain patents, enforce such patents, avoid patent infringement, and obtain patent extensions where applicable.
Government Regulation
The production and marketing of our product and our ongoing research and development activities are subject to regulation by numerous federal, provincial, state and local governmental authorities in the United States and any other countries where we may test or market our product. These laws require the approval of manufacturing facilities, including adhering to “good manufacturing” and/or “good laboratory” practices during production and storage, the controlled research and testing of products, governmental review and approval of submissions requiring manufacturing, pre-clinical and clinical data to establish the safety and efficacy of the product for each
14
use sought in order to obtain marketing approval, and the control of marketing activities, including advertising and labeling. Failure to adhere to these requirements could invalidate our data.
If we secure regulatory approval, we would continue to be subject to extensive ongoing regulatory requirements. Manufacturing of approved drug products must comply with extensive regulations governing GMP. Manufacturers and their facilities are subject to continual review and periodic inspections. As we may be dependent on third parties for manufacturing, we will have limited ability to ensure that any entity manufacturing products on our behalf is doing so in compliance with applicable GMP requirements. Failure or delay by any manufacturer of our product to comply with GMP regulations or to satisfy regulatory inspections could have a material adverse effect on us, including potentially preventing us from being able to supply products for clinical trials or commercial sales. In addition, manufacturers may need to obtain approval from regulatory authorities for product, manufacturing, or labeling changes, which requires time and money to obtain and can cause delays in product availability. We are also required to comply with good distribution practices such as maintenance of storage and shipping conditions, as well as security of products, in order to ensure product quality determined by GMP is maintained throughout the distribution network. In addition, we are subject to regulations governing the import and export of our products.
Sales and marketing of pharmaceutical products are subject to extensive federal and provincial or state laws governing on-label and off-label advertising, scientific/educational grants, gifts, consulting and pricing and are also subject to consumer protection and unfair competition laws. Compliance with extensive regulatory and enforcement requirements requires training and monitoring of the sales force and other field personnel, which could impose a substantial cost on us. To the extent our product is marketed by collaborators, our ability to ensure their compliance with applicable regulations would be limited. In addition, we are subject to regulations governing the design, testing, control, manufacturing, distribution, labeling, quality assurance, packaging, storage, shipping, import and export of our product candidate.
There can be no assurance that we will be able to achieve or maintain regulatory compliance with respect to all or any part of our current or future products or that we will be able to timely and profitably produce our product while complying with applicable regulatory requirements. If we fail to maintain compliance, regulatory authorities may not allow the continuation of the drug development programs, or require us to make substantial changes to the drug. Any such actions could have a material adverse effect on the business, financial condition, and results of operations.
Product Development Goals and Time Frames
We set goals for, and make public statements regarding, timing of the accomplishment of objectives material to our success, such as the commencement and completion of clinical trials, anticipated regulatory approval dates, and time of product launch. The actual timing of these events can vary dramatically due to factors such as delays or failures in clinical trials, the uncertainties inherent in the regulatory approval process, and delays in achieving product development, manufacturing, or marketing milestones necessary to commercialize our product. There can be no assurance that our clinical trials will be completed, that regulatory submissions will be made or receive regulatory approvals as planned, or that we will be able to adhere to the current schedule for the validation of manufacturing and launch of our product. If we fail to achieve one or more of these milestones as planned, the price of the Common Shares could decline.
We will have significant additional future capital needs in 2020 and beyond and there may be uncertainties as to our ability to raise additional funding in the future to meet those needs.
We will require significant additional capital resources to expand our business, in particular the further development of our product candidate, voclosporin, whether for LN or any other indication. Advancing our product candidate, marketing for our product, or acquisition and development of any new products or product candidates will require considerable resources and additional access to capital markets. In addition, our future
15
cash requirements may vary materially from those now expected. For example, our future capital requirements may increase if:
| • | we experience unexpected or increased costs relating to preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, or other lawsuits, brought by either us or our competition; |
| • | we experience scientific progress sooner than expected in our discovery, research and development projects, if we expand the magnitude and scope of these activities, or if we modify our focus as a result of our discoveries; |
| • | we are required to perform additional pre-clinical studies and clinical trials; or |
| • | we elect to develop, acquire or license new technologies, products or businesses. |
We could potentially seek additional funding through corporate collaborations and licensing arrangements or through public or private equity or debt financing. However, if capital market conditions in general, or with respect to life sciences companies such as ours, are unfavourable, our ability to obtain significant additional funding on acceptable terms, if at all, will be negatively affected. Additional financing that we may pursue may involve the sale of Common Shares which could result in significant dilution to our shareholders. If sufficient capital is not available, we may be required to delay our research and development projects, which could have a material adverse effect on our business, financial condition, prospects or results of operations.
Patents and Proprietary Technology
Patents and other proprietary rights are essential to our business. Our policy has been to file patent applications to protect technology, inventions, and improvements to our inventions that are considered important to the development of our business.
Our success will depend in part on our ability to obtain patents, defend patents, maintain trade secret protection and operate without infringing on the proprietary rights of others. Interpretation and evaluation of pharmaceutical patent claims present complex and often novel legal and factual questions. Accordingly, there is some question as to the extent to which biopharmaceutical discoveries and related products and processes can be effectively protected by patents. As a result, there can be no assurance that:
| • | patent applications will result in the issuance of patents; |
| • | additional proprietary products developed will be patentable; |
| • | patents issued will provide adequate protection or any competitive advantages; |
| • | patents issued will not be successfully challenged by third parties; |
| • | our products do not infringe the patents or intellectual property of others; or |
| • | that we will be able to obtain any extensions of the patent term. |
A number of pharmaceutical, biotechnology, medical device companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to our business. Some of these technologies, applications or patents may conflict with or adversely affect our technologies or intellectual property rights. Any conflicts with the intellectual property of others could limit the scope of the patents, if any, that we may be able to obtain or result in the denial of patent applications altogether.
Further, there may be uncertainty as to whether we may be able to successfully defend any challenge to our patent portfolio. Moreover, we may have to participate in interference proceedings in the various jurisdictions around the world. An unfavorable outcome in an interference or opposition proceeding or a conflict with the intellectual property of others could preclude us or our collaborators or licensees from making, using or selling
16
products using the technology, or require us to obtain license rights from third parties. It is not known whether any prevailing party would offer a license on commercially acceptable terms, if at all. Further, any such license could require the expenditure of substantial time and resources and could harm our business. If such licenses are not available, we could encounter delays or prohibition of the development or introduction of our product.
Clinical trials for our product candidate are expensive and time-consuming, and their outcome is uncertain.
Before we can obtain regulatory approval for the commercial sale of any product candidate currently under development, we are required to complete extensive clinical trials to demonstrate its safety and efficacy. Clinical trials are very expensive and difficult to design and implement. The clinical trial process is also time-consuming. If we find a collaboration partner for the development of voclosporin (whether for LN or any other indication), the clinical trials are expected to continue for several years, although costs associated with voclosporin may well be shared with our collaboration partner. The timing of the commencement, continuation and completion of clinical trials may be subject to significant delays relating to various causes, including:
| • | our inability to find collaboration partners, if needed; |
| • | our inability to manufacture or obtain sufficient quantities of materials for use in clinical trials; |
| • | delays in obtaining regulatory approvals to commence a study, or government intervention to suspend or terminate a study; |
| • | delays, suspension, or termination of the clinical trials imposed by the IRB/IEC responsible for overseeing the study to protect research subjects at a particular study site; |
| • | delays in identifying and reaching agreement on acceptable terms with prospective clinical trial sites; |
| • | slower than expected rates of patient recruitment and enrollment; |
| • | uncertain dosing issues; |
| • | inability or unwillingness of medical investigators to follow our clinical protocols; |
| • | variability in the number and types of subjects available for each study and resulting difficulties in identifying and enrolling subjects who meet trial eligibility criteria; |
| • | scheduling conflicts with participating clinicians and clinical institutions; |
| • | difficulty in maintaining contact with subjects after treatment, which results in incomplete data; |
| • | unforeseen safety issues or side effects; |
| • | lack of efficacy during the clinical trials; |
| • | our reliance on clinical research organizations to conduct clinical trials, which may not conduct those trials with good clinical or laboratory practices; or |
| • | other regulatory delays. |
The results of pre-clinical studies and initial clinical trials are not necessarily predictive of future results, and our current product candidate may not have favourable results in later trials or in the commercial setting.
Success in pre-clinical or animal studies and early clinical trials neither ensure that later large-scale efficacy trials will be successful nor does it predict final results. Pre-clinical tests and Phase 1 and Phase 2 clinical trials are primarily designed to test safety, to study pharmacokinetics and pharmacodynamics and to understand the side effects of product candidates at various doses and schedules. Favourable results in early trials may not be repeated in later trials.
A number of companies in the life sciences industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Clinical results are frequently susceptible to varying interpretations
17
that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events and could cause a clinical trial to be repeated or terminated. Pre-clinical data and the clinical results we have obtained for voclosporin (for LN or any other indication) may not predict results from studies in larger numbers of subjects drawn from more diverse populations or in a commercial setting, and also may not predict the ability of our product to achieve its intended goals, or to do so safely.
We will be required to demonstrate in Phase 3 clinical trials that voclosporin is safe and effective for use in a diverse population before we can seek regulatory approvals for its commercial sale. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical and post-approval trials. If voclosporin fails to demonstrate sufficient safety and efficacy in ongoing or future clinical trials, we could experience potentially significant delays in, or be required to abandon development of, our product candidate currently under development.
Our industry is subject to health and safety risks.
While we take substantial precautions such as laboratory and clinical testing, toxicology studies, quality control and assurance testing and controlled production methods, the health and safety risks associated with producing a product for human ingestion cannot be eliminated. Products produced by us may be found to be, or to contain substances that are harmful to the health of our patients and customers and which, in extreme cases, may cause serious health conditions or death. This sort of finding may expose us to substantial risk of litigation and liability.
Further, we would be forced to discontinue production of our product, which would harm our profitability. We maintain product liability insurance coverage; however, there is no guarantee that our current coverage will be sufficient or that we can secure insurance coverage in the future at commercially viable rates or with the appropriate limits.
Our product may not achieve or maintain expected levels of market acceptance, which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our Securities to decline.
Even if we are able to obtain regulatory approvals for our product, the success of the product is dependent upon achieving and maintaining market acceptance. New product candidates that appear promising in development may fail to reach the market or may have only limited or no commercial success. Levels of market acceptance for our product could be impacted by several factors, many of which are not within our control, including but not limited to:
| • | safety, efficacy, convenience and cost-effectiveness of our product compared to products of our competitors; |
| • | scope of approved uses and marketing approval; |
| • | timing of market approvals and market entry; |
| • | difficulty in, or excessive costs to, manufacture; |
| • | infringement or alleged infringement of the patents or intellectual property rights of others; |
| • | availability of alternative products from our competitors; |
| • | formulary placement and status; |
| • | acceptance of the price of our product; and |
| • | ability to market our product effectively at the retail level. |
18
In addition, by the time any products are ready to be commercialized, what we believe to be the market for these products may have changed. Our estimates of the number of patients who have received or might have been candidates to use a specific product may not accurately reflect the true market or market prices for such products or the extent to which such products, if successfully developed, will actually be used by patients. Our failure to successfully introduce and market our products would have a material adverse effect on our business, financial condition, and results of operations.
We are dependent upon key personnel to achieve our business objectives.
Our ability to retain key personnel and attract other qualified individuals is critical to our success. As a technology-driven company, intellectual input from key management and personnel is critical to achieve our business objectives. The loss of the services of key individuals might significantly delay or prevent achievement of our business objectives. In addition, because of a relative scarcity of individuals with experience and the high degree of education and scientific achievement required for our business, competition among life sciences companies for qualified employees is intense and, as a result, we may not be able to attract and retain such individuals on acceptable terms, or at all. In addition, because we do not maintain “key person” life insurance on any of our officers, employees, or consultants, any delay in replacing such persons, or an inability to replace them with persons of similar expertise, would have a material adverse effect on our business, financial condition, and results of operations.
We also have relationships with scientific collaborators at academic and other institutions, some of whom conduct research at our request or assist us in formulating our research and development strategies. These scientific collaborators are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, even though our collaborators are required to sign confidentiality agreements prior to working with us, they may have arrangements with other companies to assist such other companies in developing technologies that may prove competitive to us.
Incentive provisions for our key executives include the granting of stock options that vest over time, designed to encourage such individuals to stay with us. However, a low share price, whether as a result of disappointing progress in our development programs or as a result of market conditions generally, could render such agreements of little value to our key executives. In such event, our key executives could be susceptible to being hired away by our competitors who could offer a better compensation package. If we are unable to attract and retain key personnel, our business, financial conditions and results of operations may be adversely affected.
We are exposed to risks relating to the write-down of intangible assets, which comprises a significant portion of our total assets.
A significant amount of our total assets relate to our intellectual property. As of September 30, 2017, the carrying value of our intangible assets was approximately US$14.5 million. In accordance with IFRS, we are required to review the carrying value of its intangible assets for impairment periodically or when certain triggers occur. Such impairment will result in a write-down of the intangible asset and the write-down is charged to income during the period in which the impairment occurs. The write-down of any intangible assets could have a material adverse effect on our business, financial condition, and results of operations.
If we were to lose our foreign private issuer status under U.S. federal securities laws, we would likely incur additional expenses associated with compliance with the U.S. securities laws applicable to U.S. domestic issuers.
As a foreign private issuer, as defined in Rule 3b-4 under the Exchange Act, we are exempt from certain of the provisions of the U.S. federal securities laws. For example, the U.S. proxy rules and the Section 16 reporting and “short swing” profit rules do not apply to foreign private issuers. However, if we were to lose our status as a foreign private issuer, these regulations would immediately apply and we would also be required to commence
19
reporting on forms required of U.S. companies, such as Forms 10-K, 10-Q and 8-K, rather than the forms currently available to us, such as Forms 40-F and 6-K. Compliance with these additional disclosure and timing requirements under these securities laws would likely result in increased expenses and would require our management to devote substantial time and resources to comply with new regulatory requirements. Further, to the extent that we were to offer or sell our Securities outside of the United States, we would have to comply with the more restrictive Regulation S requirements that apply to U.S. companies, and we would no longer be able to utilize the multijurisdictional disclosure system forms for registered offerings by Canadian companies in the United States, which could limit our ability to access the capital markets in the future.
Legislative actions, potential new accounting pronouncements, and higher insurance costs are likely to impact our future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected revenue fluctuations and affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with greater frequency and are expected to occur in the future. Compliance with changing regulations of corporate governance and public disclosure may result in additional expenses. All of these uncertainties are leading generally toward increasing insurance costs, which may adversely affect our business, results of operations and our ability to purchase any such insurance, at acceptable rates or at all, in the future.
We rely on third parties for the supply and manufacture of voclosporin, which can be unpredictable in terms of quality, cost, timing and availability.
Our drug, voclosporin, requires a specialized manufacturing process. Lonza is currently the sole source manufacturer of voclosporin.
We have contracted Catalent to encapsulate and package voclosporin for our AURORA clinical trial program. Catalent is currently the sole supplier for encapsulating and packaging our clinical drug supply. It is our intention that Catalent will provide services with respect to encapsulating the voclosporin required for future clinical and commercial supply needs, while the provider of packaging services for commercial supply is yet to be determined.
The FDA and other regulatory authorities require that drugs be manufactured in accordance with the current good manufacturing practices regulations, as established from time to time. Accordingly, in the event we receive marketing approvals for voclosporin, it may need to rely on a limited number of third parties to manufacture and formulate voclosporin. We may not be able to arrange for our product to be manufactured on reasonable terms or in sufficient quantities.
Manufacturers of pharmaceutical products often encounter difficulties in production, especially in scaling up initial production. These problems include difficulties with production costs and yields, stability, quality control and assurance, and shortages of qualified personnel, as well as compliance with strictly enforced federal, provincial and foreign regulations. We rely on a limited number of third parties to manufacture and supply raw materials for our product. The third parties we choose to manufacture and supply raw materials for our product are not under our control, and may not perform as agreed or may terminate their agreements with us, and we may not be able to find other third parties to manufacture and supply raw materials on commercially reasonable terms, or at all. If either of these events were to occur, our operating results and financial condition would be adversely affected.
In addition, drug and chemical manufacturers are subject to various regulatory inspections, including those conducted by the FDA, to ensure strict compliance with GMP and other government regulations. While we are obligated to audit the performance of our third-party contractors, we do not have complete control over their compliance. We could be adversely impacted if our third-party manufacturers do not comply with these standards
20
and regulations. For non-compliance, the regulatory authority may levy penalties and sanctions, including fines, injunctions, civil penalties, failure of the government to grant review of submissions or market approval of drugs, or cause delays, suspension or withdrawal of approvals, product seizures or recalls, operating restrictions, facility closures and criminal prosecutions. Any of this will have a material adverse impact on our business, financial condition, and results of operations.
Anticipated Revenues may be derived from Licensing Activities
We anticipate that our revenues in the future may be derived from products licensed to pharmaceutical and biotechnology companies. Accordingly, these revenues will depend, in large part, upon the success of these companies, and our operating results may fluctuate substantially due to reductions and delays in their research, development and marketing expenditures. These reductions and delays may result from factors that are not within our control, including:
| • | changes in economic conditions; |
| • | changes in the regulatory environment, including governmental pricing controls affecting health care and health care providers; |
| • | pricing pressures; and |
| • | other factors affecting research and development spending. |
Lack of Operating Profits
We have incurred losses and anticipate that our losses will increase as we continue the development of voclosporin and clinical trials and seek regulatory approval for the sale of our therapeutic product. There can be no assurance that we will have earnings or positive cash flow in the future.
As at December 31, 2017, we had an accumulated deficit of $351.8 million. The net operating losses over the near-term and the next several years are expected to continue as a result of initiating new clinical trials and activities necessary to support regulatory approval and commercialization of our product. There can be no assurance that we will be able to generate sufficient product revenue to become profitable at all or on a sustained basis. We expect to have quarter-to-quarter fluctuations in expenses, some of which could be significant, due to research, development, and clinical trial activities, as well as regulatory and commercialization activities.
Negative Cash Flow
We had negative operating cash flow for the financial year ended December 31, 2017. We anticipate that we will continue to have negative cash flow as we continue our development of voclosporin. To the extent that we have negative operating cash flow in future periods, we will likely need to allocate a portion of our cash reserves to fund such negative cash flow. We may also be required to raise additional funds through the issuance of equity or debt securities. There can be no assurance that we will be able to generate a positive cash flow from our operations, that additional capital or other types of financing will be available when needed or that these financings will be on terms favourable or acceptable to us.
We may not realize the anticipated benefits of acquisitions or product licenses and integration of these acquisitions and any products acquired or licensed may disrupt our business and management.
As part of our business strategy, we may acquire additional companies, products or technologies principally related to, or complementary to, our current operations. At any given time, we may be evaluating new acquisitions of companies, products or technologies or may be exploring new licensing opportunities, and may have entered into confidentiality agreements, non-binding letters of intent or may be in the process of conducting
21
due diligence with respect to such opportunities. Any such acquisitions will be accompanied by certain risks including, but not limited to:
| • | exposure to unknown liabilities of acquired companies and the unknown issues with any associated technologies or research; |
| • | higher than anticipated acquisition costs and expenses; |
| • | the difficulty and expense of integrating operations, systems, and personnel of acquired companies; |
| • | disruption of our ongoing business; |
| • | inability to retain key customers, distributors, vendors and other business partners of the acquired company; |
| • | diversion of management’s time and attention; and |
| • | possible dilution to shareholders. |
We may not be able to successfully overcome these risks and other problems associated with acquisitions and this may adversely affect our business, financial condition or results of operations.
Our business depends heavily on the use of information technologies.
Several key areas of our business depend on the use of information technologies, including production, manufacturing and logistics, as well as clinical and regulatory matters. Despite our best efforts to prevent such behavior, third parties may nonetheless attempt to hack into our systems and obtain data relating to our pre-clinical studies, clinical trials, patients using our product or our proprietary information on voclosporin. If we fail to maintain or protect our information systems and data integrity effectively, we could have problems in determining product cost estimates and establishing appropriate pricing, have difficulty preventing, detecting, and controlling fraud, have disputes with physicians, and other health care professionals, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenues as a result of a data privacy breach, or suffer other adverse consequences. While we have invested in the protection of data and information technology, there can be no assurance that our efforts or those of our third-party collaborators, if any, or manufacturers, to implement adequate security and quality measures for data processing would be sufficient to protect against data deterioration or loss in the event of a system malfunction, or to prevent data from being stolen or corrupted in the event of a security breach. Any such loss or breach could have a material adverse effect on our business, operating results and financial condition.
Competition and Technological Change
The industry in which we operate is highly competitive and we have numerous domestic and foreign competitors, including major pharmaceutical and chemical companies, specialized biotechnology companies, universities, academic institutions, government agencies, public and private research organizations and large, fully-integrated pharmaceutical companies which have extensive resources and experience in research and development, process development, clinical evaluation, manufacturing, regulatory affairs, distribution and marketing. Many of our potential competitors possess substantially greater research and development skills, financial, technical and marketing expertise and human resources than we do, and may be better equipped to develop, manufacture and market products. There is a risk that new products and technologies may be developed which may be more effective or commercially viable than the product being developed or marketed by us, thus making our product non-competitive or obsolete. There may also be market resistance to the acceptance of our new product in any indication and a risk that the product, even though clinically effective, is not economically viable in the commercial production stage.
22
Reliance on Partner
Our strategy and success for the research, development, and commercialization of voclosporin in China is dependent upon the activities of a third party with rights to voclosporin in that jurisdiction. The amount and timing of resources such third party will devote to these activities may not be within our control. There can be no assurance that such third party will perform as expected.
The license, research and development agreements with the third parties referenced above include indemnification and obligation provisions that are customary in the industry. These guarantees generally require us to compensate the other party for certain damages and costs incurred as a result of third party claims or damages arising from these transactions. These provisions may survive termination of the underlying agreement. The nature of the potential obligations prevents us from making a reasonable estimate of the maximum potential amount we could be required to pay.
Reliance on Other Third Parties
We depend on third parties for the sourcing of components or for the product itself. Furthermore, as with other pharmaceutical companies, we rely on medical institutions for testing and clinically validating our prospective product. We do not anticipate any difficulties in obtaining required components or products or any difficulties in the validation and clinical testing of our product but there is no guarantee that they will be obtained.
We currently rely on CROs for the conduct of our clinical trials. These CROs operate in accordance with good clinical management practices mandated by the regulatory authorities and are subject to regular audits by regulatory authorities and by us.
We also have arrangements for the encapsulation, packaging and labeling of voclosporin through third party suppliers. Contract manufacturers must operate in compliance with regulatory requirements. Failure to do so could result in, among other things, the disruption of product supplies.
Marketing and Distribution
We have limited experience in the sales, marketing, and distribution of pharmaceutical products. There can be no assurance that we will be able to establish sales, marketing, and distribution capabilities or make arrangements through collaborations, licensees, or others to perform such activities, or that such efforts would be successful. If we decide to market our product directly, we must either acquire or internally develop a marketing and sales force with technical expertise and provide supporting distribution capabilities. The acquisition or development of a sales and distribution infrastructure would require substantial resources, which may divert the attention of management and key personnel, and have a negative impact on product development. If we contract with third parties for the sales and marketing of our product, our revenue will be dependent on the efforts of these third parties, whose efforts may not be successful. If we fail to establish successful marketing and sales capabilities or to make arrangements with third parties, the business, financial condition and results of operations will be materially adversely affected.
Health Care Reimbursement
In both domestic and foreign markets, sales of our product, if any, will be dependent in part on the availability of reimbursement from third party payors, such as government and private commercial insurance plans. Third party payors are increasingly challenging the prices charged for medical products and services. There can be no assurance that our product will be considered cost effective by these third party payors, that reimbursement will be available or if available that the payor’s reimbursement policies will not adversely affect our ability to sell our product on a profitable basis.
23
Unauthorized Disclosure of Confidential Information
There may be an unauthorized disclosure of the significant amount of confidential information under our control. We maintain and manage confidential information relating to our technology, research and development, production, marketing and business operations and those of our collaborators, in various forms. Although we have implemented controls to protect the confidentiality of such information, there can be no assurance that such controls will be effective. Unauthorized disclosures of such information could subject us to complaints or lawsuits for damages, in Canada or other jurisdictions, or could otherwise have a negative impact on our business, financial condition, results of operations, reputation and credibility.
Use of Hazardous Materials
Drug manufacturing processes involve the controlled use of hazardous materials. We and our third party manufacturing contractors, are subject to regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although we believe that our third party manufacturers have the required safety procedures for handling and disposing of such materials and comply with the standards prescribed by such laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and such liability could exceed our resources.
Liability and Insurance
The testing, marketing and sale of human pharmaceutical products involves unavoidable risks. If we succeed in developing new pharmaceutical products, the sale of such products may expose us to potential liability resulting from the use of such products. Such liability might result from claims made directly by consumers or by regulatory agencies, pharmaceutical companies or others. The obligation to pay any product liability claim in excess of whatever insurance we are able to acquire, or the recall of any of our products, could have a material adverse effect on our business, financial condition and future prospects.
We entered into indemnification agreements with our officers and directors. The maximum potential amount of future payments required under these indemnification agreements is unlimited. However, we currently maintain director and officer liability insurance coverage of US$35 million to reduce our exposure.
Financial Instruments and Risks
We are exposed to credit risks and market risks related to changes in interest rates and foreign currency exchange, each of which could affect the value of our current assets and liabilities. We invest our cash reserves in U.S. dollar denominated, fixed rate, highly liquid and highly rated financial instruments such as treasury notes, banker acceptances, bank bonds, and term deposits. We do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to our investment portfolio, due to the short-term nature of the investments and our current ability to hold these investments to maturity.
We are exposed to financial risk related to the fluctuation of foreign currency exchange rates which could have a material effect on our future operating results or cash flows. Foreign currency risk is the risk that variations in exchange rates between the United States dollar and foreign currencies, primarily with the Canadian dollar, will affect our operating and financial results. We hold our cash reserves in US dollars and the majority of our expenses, including clinical trial costs are also denominated in US dollars, which mitigates the risk of material foreign exchange fluctuations.
24
Risks Relating to the Offering
An investment in the Securities may result in the loss of an investor’s entire investment.
An investment in the Securities is speculative and may result in the loss of an investor’s entire investment. Only potential investors who are experienced in high risk investments and who can afford to lose their entire investment should consider an investment in Aurinia.
There is currently no market through which our Securities, other than the Common Shares, may be sold.
Unless otherwise specified in an applicable Prospectus Supplement, our Warrants, Subscription Receipts, Debt Securities and Units (other than any Common Shares comprising the Units) will not be listed on any securities or stock exchanges or on any automated dealer quotation system. There is currently no market through which our Securities, other than the Common Shares, may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect the pricing of our Securities, other than the Common Shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these Securities and the extent of issuer regulation.
There is no assurance of a sufficient liquid trading market for our Common Shares in the future.
Our shareholders may be unable to sell significant quantities of Common Shares into the public trading markets without a significant reduction in the price of their Common Shares, or at all. There can be no assurance that there will be sufficient liquidity of our Common Shares on the trading market, and that we will continue to meet the listing requirements of the TSX or the NASDAQ or achieve listing on any other public listing exchange.
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our technologies or drug candidate.
In order to meet our future financing needs, we may issue a significant amount of additional Common Shares, Warrants, Subscription Receipts, Debt Securities, Units, or other equity or debt securities. The precise terms of any future financing will be determined by us and potential investors and such future financings may significantly dilute our shareholders’ percentage ownership. Additionally, if we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidate or grant licenses on terms that may not be favourable to us and/or that may reduce the value of the Common Shares.
Volatility of Share Price
The market prices for the securities of biotechnology companies, including ours, have historically been volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of any particular company.
The trading price of the Common Shares could continue to be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including the results and adequacy of our preclinical studies and clinical trials, as well as those of our collaborators, or our competitors; other evidence of the safety or effectiveness of our products or those of our competitors; announcements of technological innovations or new products by us or our competitors; governmental regulatory actions; developments with collaborators; developments (including litigation) concerning our patent or other proprietary rights of competitors; concern as to the safety of our products; period-to-period fluctuations in operating results; changes in estimates of our performance by securities analysts; market conditions for biotechnology stocks in general; and other factors not within our control could have a significant adverse impact on the market price of the Common Shares, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A class action suit against us could result in substantial costs, potential liabilities and the diversion of management’s attention and resources.
25
There is no guarantee that an active trading market for the Common Shares will be maintained on the TSX and /or NASDAQ. Investors may not be able to sell their Common Shares quickly or at the latest market price if the trading in the Common Shares is not active.
We expect to issue Common Shares in the future. Future issuances of Common Shares, or the perception that such issuances are likely to occur, could affect the prevailing trading prices of the Common Shares. In addition, the existence of Warrants or Debt Securities with conversion features may encourage short selling by market participants.
Sales of Common Shares could cause a decline in the market price of the Common Shares. One of our major shareholders (ILJIN SNT Co., Ltd. and its affiliates) owns an aggregate of approximately 15.33% of our outstanding Common Shares as at March 23, 2018. Any sales of Common Shares by these shareholders or other existing shareholders or holders of options may have an adverse effect on our ability to raise capital and may adversely affect the market price of the Common Shares.
Future issuances of equity securities by us or sales by our existing shareholders may cause the price of the Common Shares to fall.
The market price of the Common Shares could decline as a result of issuances of Securities or sales by our existing shareholders in the market, or the perception that these sales could occur. Sales of Common Shares by shareholders might also make it more difficult for us to sell Common Shares at a time and price that we deem appropriate. With an additional sale or issuance of Common Shares, investors will suffer dilution of their voting power and may experience dilution in earnings per share.
We may have broad discretion in the use of the net proceeds of an offering of the Securities and may not use them to effectively manage our business.
We may need to exercise broad discretion over the use of the net proceeds from a future offering of Common Shares. Because of the number and variability of factors that will determine our use of such proceeds, our ultimate use might vary substantially from our planned use. Investors may not agree with how we allocate or spend the proceeds from an offering of Common Shares. We may pursue acquisitions, collaborations or clinical trials that do not result in an increase in the market value of the Common Shares, and may increase our losses.
We do not intend to pay dividends in the foreseeable future.
We have never declared or paid any dividends on the Common Shares. We intend, for the foreseeable future, to retain our future earnings, if any, to finance our commercial activities and further research and the expansion of our business. As a result, the return on an investment in Common Shares will likely depend upon any future appreciation in value, if any, and on a shareholder’s ability to sell Common Shares. The payment of future dividends, if any, will be reviewed periodically by our board of directors and will depend upon, among other things, conditions then existing including earnings, financial conditions, cash on hand, financial requirements to fund our commercial activities, development and growth, and other factors that our board of directors may consider appropriate in the circumstances.
We may be a passive foreign investment company for U.S. tax purposes, which may result in adverse tax consequences for U.S. investors.
If we are characterized as a passive foreign investment company (“PFIC”), there may be adverse tax consequences for U.S. investors. Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets are held for the production of, or produce, passive income, we would be characterized as a PFIC for U.S. federal income tax purposes. Based on the nature of our income and the value and composition of our assets, we do not believe we were a PFIC during 2017.
26
While we also do not believe we will be a PFIC for the current taxable year, because PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, there can be no assurance that we will not be a PFIC for the current or future taxable years. If we are characterized as a PFIC, our shareholders who are U.S. holders may suffer adverse tax consequences, including the treatment of gains realized on the sale of our ordinary shares as ordinary income, rather than as capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares by individuals who are U.S. holders, and the addition of interest charges to the tax on such gains and certain distributions. A U.S. shareholder of a PFIC generally may mitigate these adverse U.S. federal income tax consequences by making a “qualified electing fund” election, or, to a lesser extent, a “mark to market” election.
You may be unable to enforce actions against us, certain of our directors and officers, or the experts named in this Prospectus under U.S. federal securities laws.
As a corporation organized under the laws of Alberta, Canada, it may be difficult to bring actions under U.S federal securities law against us. Most of our directors and officers, as well as the experts named in this Prospectus, reside principally in Canada or outside of the United States. Because all or a substantial portion of our assets and the assets of these persons are located outside of the United States, it may not be possible for investors to effect service of process within the United States upon us or those persons. Furthermore, it may not be possible for investors to enforce against us or those persons in the United States, judgments obtained in U.S. courts based upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in actions based upon the civil liability provisions of the U.S. federal securities laws. Therefore, it may not be possible to enforce those actions against us, certain of our directors and officers or the experts named in this Prospectus.
Adverse capital market conditions could affect out liquidity.
Adverse capital market conditions could affect our ability to meet our liquidity needs, as well as our access to capital and cost of capital. We need additional funding to continue development of our internal pipeline and collaborations in the future. Our results of operations, financial condition, cash flows and capital position could be materially affected by disruptions in the capital markets.
27
There have been no material changes in our share and loan capital, on a consolidated basis, since the date of our most recently filed audited financial statements.
Unless we otherwise indicate in a Prospectus Supplement, we currently intend to use the net proceeds from the sale of the Securities, together with our existing capital, to fund our operations, which includes, but is not limited to, clinical development and commercial production of voclosporin (whether for LN or other indications), regulatory, pre-marketing and commercialization preparation activities for voclosporin primarily for LN but also potentially for other voclosporin indications, business development opportunities such as additional product in-licensing transactions, additional clinical trials or acquisitions of other businesses or products, capital expenditures and working capital. There may be circumstances where, on the basis of results obtained or for other sound business reasons, a re-allocation of funds may be necessary or prudent. Accordingly, we will have broad discretion in the application of the proceeds of an offering of Securities. Our ultimate use might vary substantially from what is stated in this Prospectus or a Prospectus Supplement and the actual amount that we spend in connection with each intended use of proceeds may vary significantly from the amounts specified in the applicable Prospectus Supplement and will depend on a number of factors, including those referred to under “Risk Factors” and any other factors set forth in the applicable Prospectus Supplement.
The use of proceeds allocated to working capital will be used to fund corporate, administration and business development activities in support of the research and development, clinical and commercial activities we undertake.
By the nature of our business as a development stage pharmaceutical company, we had negative operating cash flow for the financial year ended December 31, 2017. To the extent that we have negative operating cash flow in future periods, we will likely need to allocate a portion of our cash reserves to fund such negative cash flow. In addition, the net proceeds from any offering under this Prospectus may be used to fund such negative cash flow from operating activities. See “Risk Factors”.
More detailed information regarding the use of proceeds from the sale of Securities will be described in any applicable Prospectus Supplement.
Earnings coverage ratios will be provided in the applicable Prospectus Supplement(s) with respect to any offering and sale of Debt Securities pursuant to this Prospectus.
The applicable Prospectus Supplement will describe our prior sales as required in a Prospectus Supplement with respect to the issuance of Securities pursuant to such Prospectus Supplement.
28
The Common Shares are listed on the TSX in Canada (trading symbol: AUP) and on Nasdaq in the United States (trading symbol: AUPH).
The following table sets forth, for the periods indicated, the reported high and low prices (in Canadian dollars) and volume of Common Shares traded for each month on the TSX.
TSX
| Month | Price Range (CDN$) | Total Volume | ||||||||||
| High | Low | |||||||||||
| March, 2017 |
$ | 14.17 | $ | 4.80 | 12,279,153 | |||||||
| April, 2017 |
$ | 10.60 | $ | 8.65 | 2,649,488 | |||||||
| May, 2017 |
$ | 11.07 | $ | 7.75 | 2,062,784 | |||||||
| June, 2017 |
$ | 9.09 | $ | 7.81 | 962,381 | |||||||
| July, 2017 |
$ | 9.75 | $ | 7.80 | 1,418,472 | |||||||
| August, 2017 |
$ | 8.21 | $ | 7.20 | 711,171 | |||||||
| September, 2017 |
$ | 8.57 | $ | 7.50 | 978,676 | |||||||
| October, 2017 |
$ | 9.50 | $ | 7.04 | 1,613,476 | |||||||
| November, 2017 |
$ | 7.59 | $ | 6.61 | 1,693,411 | |||||||
| December, 2017 |
$ | 6.81 | $ | 5.68 | 917,741 | |||||||
| January, 2018 |
$ | 7.58 | $ | 5.70 | 1,344,258 | |||||||
| February, 2018 |
$ | 7.32 | $ | 5.98 | 854,513 | |||||||
| March 1-23, 2018(1) |
$ | 7.82 | $ | 6.49 | 848,796 | |||||||
| (1) | March 23, 2018 was the last trading day prior to the date of this Prospectus. |
The following table sets forth, for the periods indicated, the reported high and low prices (in United States dollars) and the volume of shares traded for each month on Nasdaq.
NASDAQ
| Month | Price Range (US$) | Total Volume | ||||||||||
| High | Low | |||||||||||
| March, 2017 |
$ | 10.54 | $ | 3.58 | 421,107,989 | |||||||
| April, 2017 |
$ | 7.86 | $ | 6.42 | 102,463,110 | |||||||
| May, 2017 |
$ | 8.19 | $ | 5.74 | 74,595,039 | |||||||
| June, 2017 |
$ | 6.87 | $ | 5.89 | 34,543,620 | |||||||
| July, 2017 |
$ | 7.68 | $ | 6.17 | 35,665,295 | |||||||
| August, 2017 |
$ | 6.62 | $ | 5.66 | 21,374,588 | |||||||
| September, 2017 |
$ | 7.08 | $ | 6.10 | 19,202,130 | |||||||
| October, 2017 |
$ | 7.60 | $ | 5.54 | 39,120,821 | |||||||
| November, 2017 |
$ | 5.92 | $ | 4.68 | 17,378,314 | |||||||
| December, 2017 |
$ | 5.36 | $ | 4.41 | 21,669,860 | |||||||
| January, 2018 |
$ | 6.11 | $ | 4.52 | 22,658,500 | |||||||
| February, 2018 |
$ | 5.77 | $ | 4.76 | 12,473,400 | |||||||
| March 1-23, 2018 |
$ | 5.99 | $ | 5.06 | 13,049,500 | |||||||
| (1) | March 23, 2018 was the last trading day prior to the date of this Prospectus. |
29
We are authorized to issue an unlimited number of Common Shares, without nominal or par value. As of March 23, 2018, 84,051,758 Common Shares are issued and outstanding. In addition, 6,433,181 Common Shares are reserved for issuance upon exercise of existing warrants and 7,841,690 Common Shares have been reserved for issuance pursuant to outstanding options.
The holders of Common Shares are entitled to one (1) vote per share held at meetings of shareholders, to receive such dividends as declared by us and to receive a share of our remaining property and assets upon our dissolution or winding up. The Common Shares are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such shares.
We may issue Warrants to purchase Common Shares. We may issue Warrants independently or together with other Securities, and Warrants sold with other Securities may be attached to or separate from the other Securities. Warrants may be issued under and governed by the terms of one or more warrant indentures (each a “Warrant Indenture”) between us and a warrant trustee (the “Warrant Trustee”) that we will name in the relevant Prospectus Supplement. Each Warrant Trustee will be a financial institution or trust company organized under the laws of Canada or any province thereof and authorized to carry on business as a trustee. The Warrants may also be issued without the benefit of a Warrant Indenture, under a warrant certificate (each a “Warrant Certificate”), which will itself contain the terms of the Warrants.
This summary of some of the provisions of the Warrants is not complete. The statements made in this Prospectus relating to any Warrant Indenture and Warrants to be issued under this Prospectus are summaries of certain anticipated provisions thereof and do not purport to be complete and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Warrant Indenture or Warrant Certificate. Prospective investors should refer to the applicable Warrant Indenture, as applicable, relating to the specific Warrants being offered for the complete terms of the Warrants. We will file a copy of the form of Warrant Indenture, if any, with the applicable securities regulatory authorities in Canada and the United States.
The applicable Prospectus Supplement relating to any Warrants offered by us will describe the particular terms of those Warrants and include specific terms relating to the offering. This description will include, where applicable:
| • | the designation and aggregate number of Warrants; |
| • | the price at which the Warrants will be offered; |
| • | the currency or currencies in which the Warrants will be offered; |
| • | the date on which the right to exercise the Warrants will commence and the date on which the right will expire; |
| • | the number of Common Shares that may be purchased upon exercise of each Warrant and the price at which and currency or currencies in which the Common Shares may be purchased upon exercise of each Warrant; |
| • | the designation and terms of any Securities with which the Warrants will be offered, if any, and the number of the Warrants that will be offered with each Security; |
| • | the date or dates, if any, on or after which the Warrants and the other Securities with which the Warrants will be offered will be transferable separately; |
| • | whether the Warrants will be subject to redemption and, if so, the terms of such redemption provisions; |
30
| • | whether we will issue the Warrants as global securities and, if so, the identity of the depositary of the global securities; |
| • | whether the Warrants will be listed on any exchange; |
| • | whether the Warrants will be governed by a Warrant Indenture or Warrant Certificates; |
| • | material United States and Canadian federal income tax consequences of owning the Warrants; and |
| • | any other material terms or conditions of the Warrants. |
It is the Warrant Indenture, as supplemented by any applicable supplemental indenture, or the Warrant Certificate, as the case may be, and not the summary in the applicable Prospectus Supplement, which defines the rights of a holder of Warrants. There may be other provisions in the Warrant Indenture or the Warrant Certificate, as the case may be, which are important to a purchaser of Warrants. Such purchaser of Warrants should read the Warrant Indenture or the Warrant Certificate, as the case may be, for a full description of the terms of the Warrants, the terms of which shall prevail to the extent of any inconsistency.
Rights of Holders Prior to Exercise
Prior to the exercise of their Warrants, holders of Warrants will not have any of the rights as holders of the Common Shares issuable upon exercise of the Warrants.
Global Securities
We may issue Warrants in whole or in part in the form of one or more global securities, which will be registered in the name of and be deposited with a depositary, or its nominee, each of which will be identified in the applicable Prospectus Supplement. The global securities may be in temporary or permanent form. The applicable Prospectus Supplement will describe the terms of any depositary arrangement and the rights and limitations of owners of beneficial interests in any global security. The applicable Prospectus Supplement also will describe the exchange, registration and transfer rights relating to any global security.
Modifications
If Warrants are issued pursuant to a Warrant Indenture, the Warrant Indenture will provide for modifications and alterations to the Warrants issued thereunder by way of a resolution of holders of Warrants at a meeting of such holders or a consent in writing from such holders. The number of holders of Warrants required to pass such a resolution or execute such a written consent will be specified in the Warrant Indenture.
We may amend any Warrant Indenture, Warrant Certificates and the Warrants, without the consent of the holders of the Warrants, to cure any ambiguity, to cure, correct or supplement any defective or inconsistent provision, or in any other manner that will not materially and adversely affect the interests of holders of outstanding Warrants.
31
DESCRIPTION OF SUBSCRIPTION RECEIPTS
We may issue Subscription Receipts, which will entitle holders to receive, upon satisfaction of certain release conditions and for no additional consideration, Common Shares, Warrants, Debt Securities or any combination thereof. Subscription Receipts will be issued pursuant to one or more subscription receipt agreements (each, a “Subscription Receipt Agreement”), each to be entered into between us and an Escrow Agent (the “Escrow Agent”), which will establish the terms and conditions of the Subscription Receipts. Each Escrow Agent will be a financial institution or trust company organized under the laws of Canada or a province thereof and authorized to carry on business as a trustee. We will file a copy of the form of Subscription Receipt Agreement with the applicable securities regulatory authorities in Canada and the United States.
The following description sets forth certain general terms and provisions of Subscription Receipts and is not intended to be complete. The statements made in this Prospectus relating to any Subscription Receipt Agreement and Subscription Receipts to be issued thereunder are summaries of certain anticipated provisions thereof and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Subscription Receipt Agreement and the Prospectus Supplement describing such Subscription Receipt Agreement.
The Prospectus Supplement relating to any Subscription Receipts we offer will describe the Subscription Receipts and include specific terms relating to their offering. All such terms will comply with the requirements of the TSX and NASDAQ relating to Subscription Receipts. If underwriters or agents are used in the sale of Subscription Receipts, one or more of such underwriters or agents may also be parties to the Subscription Receipt Agreement governing the Subscription Receipts sold to or through such underwriters or agents.
General
The Prospectus Supplement and the Subscription Receipt Agreement for any Subscription Receipts we offer will describe the specific terms of the Subscription Receipts and may include, but are not limited to, any of the following:
| • | the designation and aggregate number of Subscription Receipts offered; |
| • | the price at which the Subscription Receipts will be offered; |
| • | the currency or currencies in which the Subscription Receipts will be offered; |
| • | the designation, number and terms of the Common Shares, Warrants, Debt Securities or combination thereof to be received by holders of Subscription Receipts upon satisfaction of the release conditions, and the procedures that will result in the adjustment of those numbers; |
| • | the conditions (the “Release Conditions”) that must be met in order for holders of Subscription Receipts to receive for no additional consideration Common Shares, Warrants, Debt Securities or combination thereof; |
| • | the procedures for the issuance and delivery of Common Shares, Warrants, Debt Securities or combination thereof to holders of Subscription Receipts upon satisfaction of the Release Conditions; |
| • | whether any payments will be made to holders of Subscription Receipts upon delivery of the Common Shares, Warrants, Debt Securities or combination thereof upon satisfaction of the Release Conditions (e.g., an amount equal to the dividends we declare on Common Shares to holders of record of Common Shares during the period from the date of issuance of the Subscription Receipts to the date of issuance of any Common Shares pursuant to the terms of the Subscription Receipt Agreement); |
| • | the terms and conditions under which the Escrow Agent will hold all or a portion of the gross proceeds from the sale of Subscription Receipts, together with interest and income earned thereon (collectively, the “Escrowed Funds”), pending satisfaction of the Release Conditions; |
32
| • | the terms and conditions pursuant to which the Escrow Agent will hold Common Shares, Warrants, Debt Securities or combination thereof pending satisfaction of the Release Conditions; |
| • | the terms and conditions under which the Escrow Agent will release all or a portion of the Escrowed Funds to us upon satisfaction of the Release Conditions; |
| • | if the Subscription Receipts are sold to or through underwriters or agents, the terms and conditions under which the Escrow Agent will release a portion of the Escrowed Funds to such underwriters or agents in payment of all or a portion of their fees or commission in connection with the sale of the Subscription Receipts; |
| • | procedures for the refund by the Escrow Agent to holders of Subscription Receipts of all or a portion of the subscription price for their Subscription Receipts, plus any pro rata entitlement to interest earned or income generated on such amount, if the Release Conditions are not satisfied; |
| • | any contractual right of rescission to be granted to initial purchasers of Subscription Receipts in the event this Prospectus, the Prospectus Supplement under which Subscription Receipts are issued or any amendment hereto or thereto contains a misrepresentation; |
| • | any entitlement we may have to purchase the Subscription Receipts in the open market by private agreement or otherwise; |
| • | whether we will issue the Subscription Receipts as global securities and, if so, the identity of the depositary for the global securities; |
| • | whether we will issue the Subscription Receipts as bearer securities, registered securities or both; |
| • | provisions as to modification, amendment or variation of the Subscription Receipt Agreement or any rights or terms attaching to the Subscription Receipts; |
| • | the identity of the Escrow Agent; |
| • | whether the Subscription Receipts will be listed on any exchange; |
| • | material United States and Canadian federal tax consequences of owning the Subscription Receipts; and |
| • | any other terms of the Subscription Receipts. |
The holders of Subscription Receipts will not be shareholders. Holders of Subscription Receipts are entitled only to receive Common Shares, Warrants, Debt Securities or combination thereof on exchange of their Subscription Receipts, plus any cash payments provided for under the Subscription Receipt Agreement, if the Release Conditions are satisfied. If the Release Conditions are not satisfied, holders of Subscription Receipts shall be entitled to a refund of all or a portion of the subscription price therefor and all or a portion of the pro rata share of interest earned or income generated thereon, as provided in the Subscription Receipt Agreement.
It is the Subscription Receipt Agreement, and not the summary in the applicable Prospectus Supplement, which defines the rights of a holder of Subscription Receipts. There may be other provisions in the Subscription Receipt Agreement which are important to a purchaser of Subscription Receipts. Such purchaser of Subscription Receipts should read the Subscription Receipt Agreement for a full description of the terms of the Subscription Receipts, the terms of which shall prevail to the extent of any inconsistency.
Escrow
The Escrowed Funds will be held in escrow by the Escrow Agent, and such Escrowed Funds will be released to us (and, if the Subscription Receipts are sold to or through underwriters or agents, a portion of the Escrowed Funds may be released to such underwriters or agents in payment of all or a portion of their fees in connection with the sale of the Subscription Receipts) at the time and under the terms specified by the Subscription Receipt
33
Agreement. If the Release Conditions are not satisfied, holders of Subscription Receipts will receive a refund of all or a portion of the subscription price for their Subscription Receipts plus their pro-rata entitlement to interest earned or income generated on such amount, in accordance with the terms of the Subscription Receipt Agreement. Common Shares, Warrants or Debt Securities may be held in escrow by the Escrow Agent, and will be released to the holders of Subscription Receipts following satisfaction of the Release Conditions at the time and under the terms specified in the Subscription Receipt Agreement.
Rescission
The Subscription Receipt Agreement will also provide that any misrepresentation in this Prospectus, the Prospectus Supplement under which the Subscription Receipts are offered, or any amendment thereto, will entitle each initial purchaser of Subscription Receipts to a contractual right of rescission following the issuance of the Common Shares, Warrants or Debt Securities to such purchaser entitling such purchaser to receive the amount paid for the Subscription Receipts upon surrender of the Common Shares or Warrants, provided that such remedy for rescission is exercised in the time stipulated in the Subscription Receipt Agreement. This right of rescission does not extend to holders of Subscription Receipts who acquire such Subscription Receipts from an initial purchaser, on the open market or otherwise, or to initial purchasers who acquire Subscription Receipts in the United States.
Global Securities
We may issue Subscription Receipts in whole or in part in the form of one or more global securities, which will be registered in the name of and be deposited with a depositary, or its nominee, each of which will be identified in the applicable Prospectus Supplement. The global securities may be in temporary or permanent form. The applicable Prospectus Supplement will describe the terms of any depositary arrangement and the rights and limitations of owners of beneficial interests in any global security. The applicable Prospectus Supplement also will describe the exchange, registration and transfer rights relating to any global security.
Modifications
The Subscription Receipt Agreement will provide for modifications and alterations to the Subscription Receipts issued thereunder by way of a resolution of holders of Subscription Receipts at a meeting of such holders or a consent in writing from such holders. The number of holders of Subscriptions Receipts required to pass such a resolution or execute such a written consent will be specified in the Subscription Receipt Agreement.
34
DESCRIPTION OF DEBT SECURITIES
We may issue Debt Securities under this Prospectus. Debt Securities may be issued under and governed by the terms of one or more indentures (each a “Debt Indenture”) between us and a trustee (the “Debt Trustee”) that we will name in the relevant Prospectus Supplement. Each Debt Trustee will be a financial institution or trust company organized under the laws of Canada or any province thereof and authorized to carry on business as a trustee. Debt Securities may also be issued without the benefit of a Debt Indenture, pursuant to separate certificates (each, a “Debt Certificate”).
This summary of some of the provisions of the Debt Securities is not complete. The statements made in this Prospectus relating to any Debt Indenture, Debt Certificate and Debt Securities to be issued under this Prospectus are summaries of certain anticipated provisions thereof and do not purport to be complete and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Debt Indenture. Prospective investors should refer to the Debt Indenture or Debt Certificate relating to the specific Debt Securities being offered for the complete terms of the Debt Securities. We will file a copy of the form of Debt Indenture, if any, with the applicable securities regulatory authorities in Canada and the United States.
Each Debt Indenture or Debt Certificate, as the case may be, may provide that Debt Securities may be issued thereunder up to the aggregate principal amount which we may authorized from time to time. Any Prospectus Supplement for Debt Securities supplementing this Prospectus will contain the terms and conditions and other information with respect to the Debt Securities being offered thereby, which may include the following:
| • | the designation, aggregate principal amount, authorized denominations and ranking of such Debt Securities; |
| • | the currency or currency units for which the Debt Securities may be purchased and the currency or currency unit in which the principal and any interest is payable (in either case, if other than United States dollars); |
| • | the percentage of the principal amount at which such Debt Securities will be issued; |
| • | the date or dates on which such Debt Securities will mature; |
| • | the rate or rates per annum at which such Debt Securities will bear interest (if any), or the method of determination of such rates (if any); |
| • | the dates on which any such interest will be payable and the record dates for such payments; |
| • | the place or places where principal, premium and interest will be payable; |
| • | the Debt Trustee under any Debt Indenture pursuant to which the Debt Securities are to be issued, as applicable; |
| • | any redemption term or terms under which such Debt Securities may be defeased; |
| • | whether such Debt Securities are to be issued in registered form, “book-entry only” form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| • | any exchange or conversion terms; |
| • | any terms relating to the modification, amendment or waiver of any terms of such Debt Securities or the applicable indenture; |
| • | whether the Debt Securities are to be governed by a Debt Indenture or Debt Certificates; |
| • | the ratings, if any, issued by rating agencies; and |
| • | any other specific terms. |
35
Debt Securities may, at our option, be issued in fully registered form, in bearer form or in “book-entry only” form. Debt Securities in registered form will be exchangeable for other Debt Securities of the same series and tenor, registered in the same name, for a like aggregate principal amount in authorized denominations and will be transferable at any time or from time to time at the corporate trust office of the Debt Trustee for the Debt Securities. No charge will be made to the holder for any such exchange or transfer, except for any tax or government charge incidental thereto.
Debt Securities of a single series may be issued at various times with different maturity dates, may bear interest at different rates and may otherwise vary.
We will summarize in the applicable Prospectus Supplement certain terms of the Debt Securities being offered thereby and the relevant Debt Indenture or Debt Certificate, as the case may be, which we believe will be most important to an investor’s decision to invest in the Debt Securities being offered. It is the Debt Indenture, as supplemented by any applicable supplemental indenture, or the Debt Certificate, as the case may be, and not the summary in the applicable Prospectus Supplement, which defines the rights of a holder of Debt Securities. There may be other provisions in the Debt Indenture or the Debt Certificate, as the case may be, which are important to a purchaser of Debt Securities. Such purchaser of Debt Securities should read the Debt Indenture or the Debt Certificate, as the case may be, for a full description of the terms of the Debt Securities, the terms of which shall prevail to the extent of any inconsistency.
36
Units are a security comprised of more than one of the other Securities described in this Prospectus offered together as a “Unit”. A Unit is typically issued so the holder thereof is also the holder of each Security included in the Unit. Thus, the holder of a Unit will have the rights and obligations of a holder of each Security comprising the Unit. The agreement, if any, under which a Unit is issued may provide that the Securities comprising the Unit may not be held or transferred separately at any time or at any time before a specified date.
The particular terms and provisions of Units offered by any Prospectus Supplement, and the extent to which the general terms and provisions described below may apply to them, will be described in the Prospectus Supplement filed in respect of such Units. This description will include, where applicable: (i) the designation and terms of the Units and of the Securities comprising the Units, including whether and under what circumstances those Securities may be held or transferred separately; (ii) any provisions for the issuance, payment, settlement, transfer or exchange of the Units or of the Securities comprising the Units; (iii) whether the Units will be issued in registered or global form; and (iv) any other material terms and conditions of the Units.
See the descriptions of the other types of Securities that may be issued under this Prospectus for further information.
Common Shares may be sold under this Prospectus by way of a secondary offering by or for the account of certain of our securityholders. The Prospectus Supplement that we will file in connection with any offering of Common Shares by selling securityholders will include the following information:
| • | the names of the selling securityholders; |
| • | the number or amount of Common Shares owned, controlled or directed by each selling securityholder; |
| • | the number or amount of Common Shares being distributed for the account of each selling securityholder; |
| • | the number or amount of our securities to be owned by the selling securityholders after the distribution and the percentage that number or amount represents of the total number of our outstanding securities; |
| • | whether Common Shares are owned by the selling securityholders both of record and beneficially, of record only or beneficially only; |
| • | if the selling securityholder purchased the Common Shares being distributed within two years preceding the date of the Prospectus Supplement, the date or dates the selling securityholder acquired the Common Shares; and |
| • | if the selling securityholder acquired the Common Shares being distributed in the twelve months preceding the date of the Prospectus Supplement, the cost thereof to the selling securityholder in the aggregate and on a per share basis. |
37
New Issue
We may sell the Securities offered by this Prospectus:
| • | to or through underwriters, dealers, placement agents or other intermediaries; |
| • | directly to one or more purchasers, or |
| • | in connection with acquisitions by us. |
The applicable Prospectus Supplement will set forth the terms of the offering of the Securities, including:
| • | the name or names of any underwriters, dealers or other placement agents; |
| • | the purchase price of, and form of consideration for, the Securities and the proceeds to us; |
| • | any delayed delivery arrangements; |
| • | any underwriting commissions, fees, discounts, and other items constituting underwriters’ compensation; |
| • | any offering price; |
| • | any discounts or concessions allowed or re-allowed or paid to dealers; and |
| • | any securities exchanges on which the Securities may be listed. |
Only the underwriters named in a Prospectus Supplement are deemed to be underwriters in connection with the Securities offered by that Prospectus Supplement.
The Securities may be sold, from time to time in one or more transactions at a fixed price or prices which may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing market price or at negotiated prices, which prices may vary as between purchasers and during the period of distribution of the Securities, including sales in transactions that are deemed to be “at the market distributions” as defined in National Instrument 44-102 – Shelf Distributions.
Under agreements which may be entered into by us, underwriters, dealers and agents who participate in the distribution of Securities may be entitled to indemnification by us against certain liabilities, including liabilities under any applicable Canadian provincial securities legislation, or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof. The underwriters, dealers and agents with whom we enter into agreements may be customers of, engage in transactions with, or perform services for, us in the ordinary course of business.
In connection with the offering of the Securities, the underwriters may over-allot or effect transactions which stabilize or maintain the market price of the Securities offered at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. A purchaser that acquires Securities forming part of the underwriters’ over-allocation position acquires those Securities under this Prospectus, regardless of whether the over-allocation position is ultimately filled through the exercise of the over-allotment option or secondary market purchases. No underwriter or dealer involved in the distribution, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot Securities or effect any other transactions that are intended to stabilize or maintain the market price of the Securities in connection with any distribution of Securities that is an “at the market distribution.”
Secondary Offering
This Prospectus may also, from time to time, relate to the offering of Common Shares by certain selling securityholders.
38
The selling securityholders may sell all or a portion of Common Shares beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If Common Shares are sold through underwriters or broker-dealers, the selling securityholders will be responsible for underwriting discounts or commissions or agent’s commissions. Common Shares may be sold by the selling securityholders in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions, as follows:
| • | on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| • | in the over-the-counter market; |
| • | in transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| • | through the writing of options, whether such options are listed on an options exchange or otherwise; |
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | short sales; |
| • | sales pursuant to Rule 144 under the U.S. Securities Act 1933 (the “U.S. Securities Act”); |
| • | broker-dealers may agree with the selling securityholders to sell a specified number of such shares at a stipulated price per share; |
| • | a combination of any such methods of sale; and |
| • | any other method permitted pursuant to applicable law. |
If the selling securityholders effect such transactions by selling Common Shares to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling securityholders or commissions from purchasers of Common Shares for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of Common Shares or otherwise, the selling securityholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of Common Shares in the course of hedging in positions they assume. The selling securityholders may also sell Common Shares short and deliver Common Shares covered by this Prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling securityholders may also loan or pledge Common Shares to broker-dealers that in turn may sell such shares.
The selling securityholders may pledge or grant a security interest in some or all of the Common Shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell Common Shares from time to time pursuant to this Prospectus or any Prospectus Supplement filed under General Instruction II.L. of Form F-10 under the U.S. Securities Act, amending, if necessary, the list of selling securityholders to include, pursuant to a prospectus amendment or Prospectus Supplement, the pledgee, transferee or other successors in interest as selling securityholders under this Prospectus. The selling securityholders also may transfer and donate Common Shares in other circumstances in which case the transferees, donees, pledgees or other successor in interest will be the selling beneficial owners for purposes of this Prospectus.
39
The selling securityholders and any broker-dealer participating in the distribution of Common Shares may be deemed to be “underwriters” within the meaning of the U.S. Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the U.S. Securities Act. At the time a particular offering of Common Shares is made, a Prospectus Supplement, if required, will be distributed which will identify the selling securityholders and provide the other information set forth under “Selling Securityholders”, set forth the aggregate amount of Common Shares being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling securityholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
Under the securities laws of some states, Common Shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states Common Shares may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any securityholder will sell any or all of Common Shares registered pursuant to the registration statement, of which this prospectus forms a part.
The selling securityholders and any other person participating in such distribution will be subject to applicable provisions of Canadian securities legislation and the U.S. Exchange Act and the rules and regulations thereunder, including, without limitations, Regulation M under the U.S. Exchange Act, which may limit the timing of purchases and sales of any Common Shares by the selling securityholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of Common Shares to engage in market-making activities with respect to Common Shares. All of the foregoing may affect the marketability of Common Shares and the ability of any person or entity to engage in market-making activities with respect to Common Shares.
Once sold under the shelf registration statement, of which this Prospectus forms a part, Common Shares will be freely tradable in the hands of persons other than our affiliates.
40
CERTAIN INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain Canadian federal income tax consequences to an investor who is a non-resident of Canada or to an investor who is a resident of Canada of acquiring, owning or disposing of any of our Securities offered thereunder.
The applicable Prospectus Supplement may also describe certain U.S. federal income tax consequences of the acquisition, ownership and disposition of any of our Securities offered thereunder by an initial investor who is a U.S. person (within the meaning of the U.S. Internal Revenue Code), including, to the extent applicable, such consequences related to debt securities payable in a currency other than the U.S. dollar, issued at an original issue discount for U.S. federal income tax purposes or containing early redemption provisions or other special items.
The auditors of Aurinia Pharmaceuticals Inc. are PricewaterhouseCoopers LLP, Edmonton, Alberta, Canada. PricewaterhouseCoopers LLP has reported on our fiscal 2016 and 2017 audited consolidated financial statements, which have been filed with the securities regulatory authorities and incorporated herein. PricewaterhouseCoopers LLP is independent with respect to us within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of Alberta and with respect to SEC auditor independence rules.
TRANSFER AGENTS AND REGISTRARS
The co-transfer agents and co-registrars of Aurinia Pharmaceuticals Inc. are Computershare Investor Services Inc. located at its principal offices in Calgary, Alberta and Toronto, Ontario and Computershare Trust Company, N.A. located at its principal offices in Golden, Colorado.
Hyuek Joon Lee, David Jayne, George Milne, Lorin Jeffry Randall and Joseph Hagan are our directors and reside outside of Canada. Each of these directors has appointed the following agent for service of process in Canada:
| Name of Person |
Name and Address of Agent | |
| Hyuek Joon Lee, David Jayne, George Milne, Lorin Jeffry Randall and Joseph Hagan | Borden Ladner Gervais LLP 1200 Waterfront Centre 200 Burrard Street, P.O. Box 48600 Vancouver, BC V7X 1T2 Attention : Stephen P. Robertson |
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
41
Certain Canadian legal matters relating to the Securities offered by this Prospectus will be passed upon for us by Borden Ladner Gervais LLP, Vancouver, British Columbia. The partners and associates of Borden Ladner Gervais LLP, Vancouver, British Columbia beneficially own, directly or indirectly, less than 1% of the Common Shares issued by Aurinia Pharmaceuticals Inc.
WHERE CAN YOU FIND MORE INFORMATION
We are required to file with the securities commission or authority in each of the applicable provinces and territories of Canada annual and quarterly reports, material change reports and other information. In addition, we are subject to the informational requirements of the Exchange Act, and, in accordance with the Exchange Act, we also file reports with, and furnish other information to, the SEC. Under a multijurisdictional disclosure system adopted by the United States and Canada, these reports and other information (including financial information) may be prepared in accordance with the disclosure requirements of Canada, which differ in certain respects from those in the United States. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required to publish financial statements as promptly as U.S. companies.
You may read any document we file with or furnish to the securities commissions and authorities of the applicable provinces of Canada through SEDAR and any document we file with, or furnish to, the SEC at the SEC’s public reference room at Station Place, 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms. Certain of our filings are also electronically available on EDGAR, and may be accessed at www.sec.gov.
ENFORCEABILITY OF CIVIL LIABILITIES
We are a corporation existing under the Business Corporations Act (Alberta). Most of our directors and officers, and the experts named in this Prospectus, are residents of Canada or otherwise reside outside the United States, and all or a substantial portion of their assets may be, and a substantial portion of the Corporation’s assets are, located outside the United States. We have appointed an agent for service of process in the United States (as set forth below) but it may be difficult for holders of securities who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. We have been advised that a judgment of a U.S. court predicated solely upon civil liability under U.S. federal securities laws or the securities of “blue sky” laws of any state within the United States, would likely be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. We have also been advised, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of the liability predicated solely upon U.S. federal securities laws.
We filed with the SEC, concurrently with our registration statement on Form F-10 of which this Prospectus is a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, we appointed CT Corporation System, 111 Eighth Avenue, New York, New York 10011 as our agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving us in a U.S. court arising out of or related to or concerning the offering of securities under this Prospectus.
42
CANADIAN PURCHASER’S STATUTORY AND CONTRACTUAL RIGHTS
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a Prospectus or a Prospectus Supplement relating to the securities purchased by a purchaser and any amendments thereto. In several of the provinces, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revision of the price or damages if the Prospectus or a Prospectus Supplement relating to the securities purchased by a purchaser and any amendments thereto contain a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for the particulars of these rights or consult with a legal adviser.
In an offering of Securities which are convertible, exchangeable or exercisable for other securities (such as Warrants, Subscription Receipts, convertible Debt Securities or Units, as applicable) investors are cautioned that the statutory right of action for damages for a misrepresentation contained in the Prospectus is limited, in certain provincial securities legislation, to the price at which the convertible, exchangeable or exercisable Securities are offered to the public under the Prospectus offering. This means that, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon conversion or exchange of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal adviser.
Original purchasers of Securities which are convertible, exchangeable or exercisable for other securities of ours (if offered separately) will have a contractual right of rescission against us in respect of the exercise of such convertible, exchangeable or exercisable Securities. The contractual right of rescission will entitle such original purchasers to receive, in addition to the amount paid on original purchase of the applicable convertible, exchangeable or exercisable Securities the amount paid upon exercise upon surrender of the underlying securities gained thereby, in the event that this Prospectus (as supplemented or amended) contains a misrepresentation, provided that: (i) the exercise takes place within 180 days of the date of the purchase of such Securities under this Prospectus; and (ii) the right of rescission is exercised within 180 days of the date of purchase of such Securities under this Prospectus. This contractual right of rescission will be consistent with the statutory right of rescission described under section 131 of the Securities Act (British Columbia), and is in addition to any other right or remedy available to original purchasers under section 131 of the Securities Act (British Columbia) or otherwise at law.
Original purchasers are further advised that in certain provinces the statutory right of action for damages in connection with a prospectus misrepresentation is limited to the amount paid for the security that was purchased under a prospectus, and therefore a further payment at the time of exercise may not be recoverable in a statutory action for damages. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights, or consult with a legal advisor.
43
CERTIFICATE OF AURINIA PHARMACEUTICALS INC.
Dated: March 26, 2018
This short form prospectus, together with the documents incorporated in this prospectus by reference, will, as of the date of the last supplement to this prospectus relating to the securities offered by this prospectus and the supplement(s), constitute full, true and plain disclosure of all material facts relating to the securities offered by this prospectus and the supplement(s) as required by the securities legislation of British Columbia, Alberta and Ontario.
| (signed) Richard Glickman |
(signed) Dennis Bourgeault | |||
| Richard Glickman | Dennis Bourgeault | |||
| Chief Executive Officer | Chief Financial Officer |
On Behalf of the Board of Directors
| (signed) L. Jeffry Randall |
(signed) Benjamin Rovinski | |||
| L. Jeffry Randall | Benjamin Rovinski | |||
| Director | Director |
44